Dying to Defend: Neutrophil Death Pathways and their Implications in Immunity
- PMID: 38044275
- PMCID: PMC10885667
- DOI: 10.1002/advs.202306457
Dying to Defend: Neutrophil Death Pathways and their Implications in Immunity
Abstract
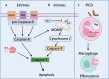
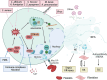
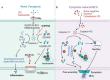
Neutrophils, accounting for ≈70% of human peripheral leukocytes, are key cells countering bacterial and fungal infections. Neutrophil homeostasis involves a balance between cell maturation, migration, aging, and eventual death. Neutrophils undergo different death pathways depending on their interactions with microbes and external environmental cues. Neutrophil death has significant physiological implications and leads to distinct immunological outcomes. This review discusses the multifarious neutrophil death pathways, including apoptosis, NETosis, pyroptosis, necroptosis, and ferroptosis, and outlines their effects on immune responses and disease progression. Understanding the multifaceted aspects of neutrophil death, the intersections among signaling pathways and ramifications of immunity will help facilitate the development of novel therapeutic methods.
Keywords: NETosis; ferroptosis; necroptosis; neutrophil apoptosis; pyroptosis.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Grants and funding
- 2022YFA0807300/National Key R&D Program of China
- 2021YFA1100600/National Key R&D Program of China
- SWY202202/Suzhou Foreign Academician Workstation
- 2017B01012/National Center for International Research-Cambridge-Su Genomic Research Center
- ZXL2021440/Suzhou Municipal Science and Technology Bureau
LinkOut - more resources
Full Text Sources
