Cerebral Small Vessel Disease, Hypertension, and Vascular Contributions to Cognitive Impairment and Dementia
- PMID: 38044814
- PMCID: PMC10734789
- DOI: 10.1161/HYPERTENSIONAHA.123.19943
Cerebral Small Vessel Disease, Hypertension, and Vascular Contributions to Cognitive Impairment and Dementia
Abstract
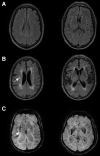
Hypertension-associated cerebral small vessel disease is a common finding in older people. Strongly associated with age and hypertension, small vessel disease is found at autopsy in over 50% of people aged ≥65 years, with a spectrum of clinical manifestations. It is the main cause of lacunar stroke and a major source of vascular contributions to cognitive impairment and dementia. The brain areas affected are subcortical and periventricular white matter and deep gray nuclei. Neuropathological sequelae are diffuse white matter lesions (seen as white matter hyperintensities on T2-weighted magnetic resonance imaging), small ischemic foci (lacunes or microinfarcts), and less commonly, subcortical microhemorrhages. The most common form of cerebral small vessel disease is concentric, fibrotic thickening of small penetrating arteries (up to 300 microns outer diameter) termed arteriolosclerosis. Less common forms are small artery atheroma and lipohyalinosis (the lesions described by C. Miller Fisher adjacent to lacunes). Other microvascular lesions that are not reviewed here include cerebral amyloid angiopathy and venous collagenosis. Here, we review the epidemiology, neuropathology, clinical management, genetics, preclinical models, and pathogenesis of hypertensive small vessel disease. Knowledge gaps include initiating factors, molecular pathogenesis, relationships between arterial pathology and tissue damage, possible reversibility, pharmacological targets, and molecular biomarkers. Progress is anticipated from multicell transcriptomic and proteomic profiling, novel experimental models and further target-finding and interventional clinical studies.
Keywords: arteries; blood pressure; dementia; genetics; neuropathology; stroke; white matter.
Conflict of interest statement
Figures



References
-
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C; Medical Research Council Cognitive Function and Ageing Study. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142 - PubMed
-
- MRC-CFAS. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3 - PubMed
-
- Oveisgharan S, Kim N, Agrawal S, Yu L, Leurgans S, Kapasi A, Arfanakis K, Bennett DA, Schneider JA, Buchman AS. Brain and spinal cord arteriolosclerosis and its associations with cerebrovascular disease risk factors in community-dwelling older adults. Acta Neuropathol. 2023;145:219–233. doi: 10.1007/s00401-022-02527-z - PMC - PubMed
-
- Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJG. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–135. doi: 10.1136/jnnp.2009.204685 - PubMed
-
- Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol. 1968;12:1–15. doi: 10.1007/BF00685305 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

