Efficacy of intravenous nalbuphine for managing post-anaesthesia shivering: A systematic review and meta-analysis of randomised controlled trials with trial sequential analysis
- PMID: 38044924
- PMCID: PMC10691608
- DOI: 10.4103/ija.ija_482_23
Efficacy of intravenous nalbuphine for managing post-anaesthesia shivering: A systematic review and meta-analysis of randomised controlled trials with trial sequential analysis
Abstract
Background and aims: Post-anaesthesia shivering is distressing and is observed after spinal and general anaesthesia. Nalbuphine, a partial mu-opioid receptor antagonist with kappa-opioid receptor agonist properties, has been successfully used to manage post-anaesthesia shivering.
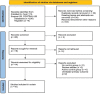
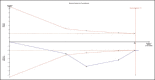
Methods: After registering the review with the International Prospective Register of Systematic Reviews (PROSPERO), we searched PubMed/Medline, Scopus, Ovid, Cochrane Library and clinicaltrials.gov with keywords for randomised controlled trials. The risk of bias-2 (RoB-2) scale was used to assess the quality of evidence. We also used Grading of Recommendations, Assessment, Development and Evaluations (GRADE) guidelines to evaluate the strength of evidence and trial sequential analysis to validate the conclusions.
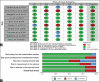
Results: Of the 240 articles, 10 were considered eligible for review (700 patients, 350- nalbuphine, 350- control or placebo). When compared to placebo, the success rate of nalbuphine controlling shivering was significantly better (risk ratio [RR]: 2.37, 95% confidence interval [CI]:1.91, 2.94; P = 0.04, I² = 94%), but comparable to the control group drugs (opioids, dexmedetomidine, ondansetron, pethidine). Compared to placebo, shivering recurrence was significantly less with nalbuphine than with placebo (RR: 0.47, 95% CI: 0.26, 0.83; P = 0.01, I² = 61%), but comparable with the control group. The incidence of postoperative nausea/vomiting (PONV) was significantly less with nalbuphine when compared to the control group (RR: 0.67, 95% CI: 0.47, 0.95; P = 0.02, I² = 37%), but PONV in the nalbuphine group was comparable to placebo (RR: 1.20, 95% CI: 0.68, 2.12; P = 0.54, I² = 0%). Other outcomes, like the grade of shivering and hypotension, were comparable between the nalbuphine and control groups.
Conclusion: Nalbuphine successfully controls post-anaesthesia shivering and reduces the recurrence of shivering.
Keywords: Anaesthesia; dexmedetomidine; meta-analysis; nalbuphine; ondansetron; opioid; shivering; systematic review.
Copyright: © 2023 Indian Journal of Anaesthesia.
Conflict of interest statement
There are no conflicts of interest.
Figures



References
-
- De Witte J, Sessler DI. Perioperative shivering: Physiology and pharmacology. Anesthesiology. 2002;96:467–84. - PubMed
-
- Choi KE, Park B, Moheet AM, Rosen A, Lahiri S, Rosengart A. Systematic quality assessment of published antishivering protocols. Anesth Analg. 2017;124:1539–46. - PubMed
-
- Park SM, Mangat HS, Berger K, Rosengart AJ. Efficacy spectrum of anti shivering medications: Meta-analysis of randomized controlled trials. Crit Care Med. 2012;40:3070–82. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
