CAR-T Cell Therapy Shows Similar Efficacy and Toxicity in Patients With DLBCL Regardless of CNS Involvement
- PMID: 38044958
- PMCID: PMC10691788
- DOI: 10.1097/HS9.0000000000000984
CAR-T Cell Therapy Shows Similar Efficacy and Toxicity in Patients With DLBCL Regardless of CNS Involvement
Abstract
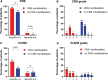
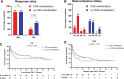
Efficacy and toxicity of chimeric antigen receptor T (CAR-T) cell therapy in relapsed/refractory (r/r) diffuse large B-cell lymphoma (DLBCL) with central nervous system (CNS) involvement remain understudied. Here we analyzed the outcomes of CAR-T cell therapy in r/r DLBCL patients with CNS involvement and compared them with patients without CNS disease. Retrospective and monocentric comparative analysis of patient cohort with r/r DLBCL treated with CAR-T cell therapy: 15 patients with CNS versus 65 patients without CNS involvement. Overall response rates (80% versus 80%; P = 1.0), progression-free survival (P = 0.157), and overall survival (P = 0.393) were comparable for both cohorts. The frequency of cytokine release syndrome was comparable in the CNS and non-CNS cohorts; 93% versus 80%; P = 1.0. Numerically, immune effector-cell-associated neurotoxicity syndrome (all grades) was more frequent in patients with CNS manifestation (53% versus 29%; P = 0.063), although no grade 4 events were documented. Our study suggests that CAR-T cell therapy is effective and feasible in patients with r/r DLBCL and CNS manifestation.
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Hematology Association.
Conflict of interest statement
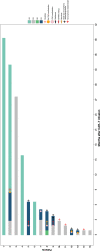
ES received honoraria from Amgen, BMS, Stemline, Sanofi, Incyte, Janssen, Takeda and JAZZ. GL received research grants not related to this article from AGIOS, AQUINOX, AstraZeneca, Bayer, Celgene, Gilead, Janssen, Morphosys, Novartis, Roche, and Verastem. GL received honoraria from ADC Therapeutics, Abbvie, Amgen, AstraZeneca, Bayer, BMS, Celgene, Constellation, Genase, Genmab, Gilead, Hexal-Sandoz, Immagene, Incyte, Janssen, Karyopharm, Lilly, Miltenyi, Morphosys, NanoString, Novartis, PentixaPharm, Roche, Sobi, and Takeda. All the other authors have no conflicts of interest to disclose.
Figures