The remote intercessory prayer, during the clinical evolution of patients with COVID -19, randomized double-blind clinical trial
- PMID: 38045114
- PMCID: PMC10689938
- DOI: 10.1016/j.heliyon.2023.e22411
The remote intercessory prayer, during the clinical evolution of patients with COVID -19, randomized double-blind clinical trial
Abstract
The objective of this study was to evaluate the effect of intercessory prayer performed by a group of spiritual leaders on the health outcomes of hospitalized patients with Novel Coronavirus (COVID-19) infection, specifically focusing on mortality and hospitalization rates.
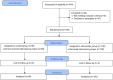
Design: This was a double-blinded, controlled, and randomized trial conducted at a private hospital in São Paulo, Brazil.
Interventions: Both groups continued to receive their usual medical care in accordance with HCor Hospital's institutional patient care protocol for COVID-19 patients.
Intervention: Both groups received their regular medical care according to HCor's institutional patient care protocol for COVID-19 patients. The intervention group, in addition to standard treatment, received intercessory prayers performed by a group of spiritual leaders.
Main outcome measures: The primary endpoint was in-hospital mortality. Secondary endpoints included the need for mechanical ventilation during hospitalization, duration of mechanical ventilation, length of ICU stay, and length of hospital stay.
Results: A total of 199 participants were randomly assigned to the groups. The primary outcome, in-hospital mortality, occurred in 8 out of 100 (8.0 %) patients in the intercessory prayer group and 8 out of 99 (8.1 %) patients in the control group (HR 0.86 [0.32 to 2.31]; p = 0.76). Additionally, there were no significant differences between the groups in terms of secondary outcomes.
Conclusion: The study found no evidence of an effect of intercessory prayer on the primary outcome of mortality or on the secondary outcomes of hospitalization time, ICU time, and mechanical ventilation time.
Keywords: COVID-19; Faith healing; Hospitalization; Prayer; Randomized clinical trials.
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Similar articles
-
A randomized, blinded study of the impact of intercessory prayer on spiritual well-being in patients with cancer.Altern Ther Health Med. 2012 Sep-Oct;18(5):18-27. Altern Ther Health Med. 2012. PMID: 22894887 Clinical Trial.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
A randomized, controlled trial of the effects of remote, intercessory prayer on outcomes in patients admitted to the coronary care unit.Arch Intern Med. 1999 Oct 25;159(19):2273-8. doi: 10.1001/archinte.159.19.2273. Arch Intern Med. 1999. PMID: 10547166 Clinical Trial.
-
Should academic medical centers conduct clinical trials of the efficacy of intercessory prayer?Acad Med. 2001 Aug;76(8):791-7. doi: 10.1097/00001888-200108000-00008. Acad Med. 2001. PMID: 11500278 Review.
-
Research on Intercessory Prayer: Theoretical and Methodological Considerations.J Relig Health. 2017 Dec;56(6):1930-1936. doi: 10.1007/s10943-015-0172-9. J Relig Health. 2017. PMID: 26743876 Review.
References
-
- Haas E.J., Angulo F.J., McLaughlin J.M., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. - DOI - PMC - PubMed
-
- Freise N.F., Kivel M., Grebe O., Meyer C., Wafaisade B., Peiper M., Zeus T., Schmidt J., Neuwahl J., Jazmati D., Luedde T., Bölke E., Feldt T., Jensen B.E.O., Bode J., Keitel V., Haussmann J., Tamaskovics B., Budach W., Fischer J.C., Knoefel W.T., Schneider M., Gerber P.A., Pedoto A., Häussinger D., van Griensven M., Rezazadeh A., Flaig Y., Kirchner J., Antoch G., Schelzig H., Matuschek C. Acute cardiac side effects after COVID-19 mRNA vaccination: a case series. Eur. J. Med. Res. 2022;27(1):80. doi: 10.1186/s40001-022-00695-y. PMID: 35655235; PMCID: PMC9160507. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources