This is a preprint.
Staphylococcus aureus skin colonization is mediated by SasG lectin variation
- PMID: 38045275
- PMCID: PMC10690190
- DOI: 10.1101/2023.11.20.567970
Staphylococcus aureus skin colonization is mediated by SasG lectin variation
Update in
-
Staphylococcus aureus skin colonization is mediated by SasG lectin variation.Cell Rep. 2024 Apr 23;43(4):114022. doi: 10.1016/j.celrep.2024.114022. Epub 2024 Apr 2. Cell Rep. 2024. PMID: 38568806 Free PMC article.
Abstract
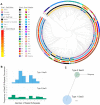
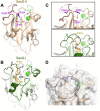
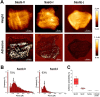
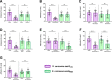
Staphylococcus aureus causes the majority of skin and soft tissue infections, but this pathogen only transiently colonizes healthy skin. However, this transient skin exposure enables S. aureus to transition to infection. Initial adhesion of S. aureus to skin corneocytes is mediated by surface protein G (SasG). Here, phylogenetic analyses reveal the presence of two major divergent SasG alleles in S. aureus, SasG-I and SasG-II. Structural analyses of SasG-II identified a unique non-aromatic arginine in the binding pocket of the lectin subdomain that mediates adhesion to corneocytes. Atomic force microscopy and corneocyte adhesion assays indicated SasG-II can bind to a broader variety of ligands than SasG-I. Glycosidase treatment resulted in different binding profiles between SasG-I and SasG-II on skin cells. Additionally, SasG-mediated adhesion was recapitulated using differentiated N/TERT keratinocytes. Our findings indicate that SasG-II has evolved to adhere to multiple ligands, conferring a distinct advantage to S. aureus during skin colonization.
Conflict of interest statement
Declaration of interests A.B.H. has served as a Scientific Advisory Board member for Hoth Therapeutics, Inc., holds equity in Hoth Therapeutics and Chelexa BioSciences, LLC, and was a co-inventor on seven patents broadly related to the subject matter of this work.
Figures
Similar articles
-
Staphylococcus aureus skin colonization is mediated by SasG lectin variation.Cell Rep. 2024 Apr 23;43(4):114022. doi: 10.1016/j.celrep.2024.114022. Epub 2024 Apr 2. Cell Rep. 2024. PMID: 38568806 Free PMC article.
-
Staphylococcal Corneocyte Adhesion: Assay Optimization and Roles of Aap and SasG Adhesins in the Establishment of Healthy Skin Colonization.Microbiol Spectr. 2022 Dec 21;10(6):e0246922. doi: 10.1128/spectrum.02469-22. Epub 2022 Oct 11. Microbiol Spectr. 2022. PMID: 36219106 Free PMC article.
-
Glycan-Dependent Corneocyte Adherence of Staphylococcus epidermidis Mediated by the Lectin Subdomain of Aap.mBio. 2021 Aug 31;12(4):e0290820. doi: 10.1128/mBio.02908-20. Epub 2021 Jul 13. mBio. 2021. PMID: 34253065 Free PMC article.
-
Interactions Between Atopic Dermatitis and Staphylococcus aureus Infection: Clinical Implications.Allergy Asthma Immunol Res. 2019 Sep;11(5):593-603. doi: 10.4168/aair.2019.11.5.593. Allergy Asthma Immunol Res. 2019. PMID: 31332972 Free PMC article. Review.
-
Host- and microbe determinants that may influence the success of S. aureus colonization.Front Cell Infect Microbiol. 2012 May 4;2:56. doi: 10.3389/fcimb.2012.00056. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919647 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
