This is a preprint.
Safety and immunogenicity of booster vaccination and fractional dosing with Ad26.COV2.S or BNT162b2 in Ad26.COV2.S-vaccinated participants
- PMID: 38045321
- PMCID: PMC10690356
- DOI: 10.1101/2023.11.20.23298785
Safety and immunogenicity of booster vaccination and fractional dosing with Ad26.COV2.S or BNT162b2 in Ad26.COV2.S-vaccinated participants
Update in
-
Safety and immunogenicity of booster vaccination and fractional dosing with Ad26.COV2.S or BNT162b2 in Ad26.COV2.S-vaccinated participants.PLOS Glob Public Health. 2024 Apr 11;4(4):e0002703. doi: 10.1371/journal.pgph.0002703. eCollection 2024. PLOS Glob Public Health. 2024. PMID: 38603677 Free PMC article.
Abstract
Background: We report the safety and immunogenicity of fractional and full dose Ad26.COV2.S and BNT162b2 in an open label phase 2 trial of participants previously vaccinated with a single dose of Ad26.COV2.S, with 91.4% showing evidence of previous SARS-CoV-2 infection.
Methods: A total of 286 adults (with or without HIV) were enrolled >4 months after an Ad26.COV2.S prime and randomized 1:1:1:1 to receive either a full or half-dose booster of Ad26.COV2.S or BNT162b2 vaccine. B cell responses (binding, neutralization and antibody dependent cellular cytotoxicity-ADCC), and spike-specific T-cell responses were evaluated at baseline, 2, 12 and 24 weeks post-boost. Antibody and T-cell immunity targeting the Ad26 vector was also evaluated.
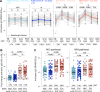
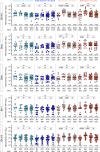
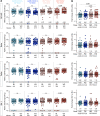
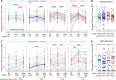
Results: No vaccine-associated serious adverse events were recorded. The full- and half-dose BNT162b2 boosted anti-SARS-CoV-2 binding antibody levels (3.9- and 4.5-fold, respectively) and neutralizing antibody levels (4.4- and 10-fold). Binding and neutralizing antibodies following half-dose Ad26.COV2.S were not significantly boosted. Full-dose Ad26.COV2.S did not boost binding antibodies but slightly enhanced neutralizing antibodies (2.1-fold). ADCC was marginally increased only after a full-dose BNT162b2. T-cell responses followed a similar pattern to neutralizing antibodies. Six months post-boost, antibody and T-cell responses had waned to baseline levels. While we detected strong anti-vector immunity, there was no correlation between anti-vector immunity in Ad26.COV2.S recipients and spike-specific neutralizing antibody or T-cell responses post-Ad26.COV2.S boosting.
Conclusion: In the context of hybrid immunity, boosting with heterologous full- or half-dose BNT162b2 mRNA vaccine demonstrated superior immunogenicity 2 weeks post-vaccination compared to homologous Ad26.COV2.S, though rapid waning occurred by 12 weeks post-boost.
Trial registration: South African National Clinical Trial Registry (SANCR): DOH-27-012022-7841.
Funding: South African Medical Research Council (SAMRC) and South African Department of Health (SA DoH).
Conflict of interest statement
Declaration of interest: A.Se. is a consultant for AstraZeneca Pharmaceuticals, Calyptus Pharmaceuticals, Inc, Darwin Health, EmerVax, EUROIMMUN, F. Hoffman-La Roche Ltd, Fortress Biotech, Gilead Sciences, Granite bio., Gritstone Oncology, Guggenheim Securities, Moderna, Pfizer, RiverVest Venture Partners, and Turnstone Biologics. A.G. is a consultant for Pfizer. LJI has filed for patent protection for various aspects of T cell epitope and vaccine design work. All other authors declare no competing interests.
Figures






References
-
- Bekker LG, Garrett N, Goga A, Fairall L, Reddy T, Yende-Zuma N, et al. Effectiveness of the Ad26.COV2.S vaccine in health-care workers in South Africa (the Sisonke study): results from a single-arm, open-label, phase 3B, implementation study. Lancet. 2022;399(10330):1141–53. doi: 10.1016/S0140-6736(22)00007-1. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
