This is a preprint.
Animal septins contain functional transmembrane domains
- PMID: 38045322
- PMCID: PMC10690161
- DOI: 10.1101/2023.11.20.567915
Animal septins contain functional transmembrane domains
Update in
-
Animal septins contain functional transmembrane domains.Curr Biol. 2025 Apr 21;35(8):1910-1917.e5. doi: 10.1016/j.cub.2025.03.004. Epub 2025 Mar 28. Curr Biol. 2025. PMID: 40157353
Abstract
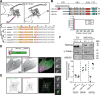
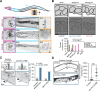
Septins, a conserved family of filament-forming proteins, contribute to eukaryotic cell division, polarity, and membrane trafficking. Septins scaffold other proteins to cellular membranes, but it is not fully understood how septins associate with membranes. We identified and characterized an isoform of Caenorhabditis elegans septin UNC-61 that was predicted to contain a transmembrane domain (TMD). The TMD isoform is expressed in a subset of tissues where the known septins were known to act, and TMD function was required for tissue integrity of the egg-laying apparatus. We found predicted TMD-containing septins across much of opisthokont phylogeny and demonstrated that the TMD-containing sequence of a primate TMD-septin is sufficient for localization to cellular membranes. Together, our findings reveal a novel mechanism of septin-membrane association with profound implications for septin dynamics and regulation.
Conflict of interest statement
Competing interests: The authors have declared no competing interests.
Figures

References
-
- Tanaka-Takiguchi Y., Kinoshita M. & Takiguchi K. Septin-Mediated Uniform Bracing of Phospholipid Membranes. Current Biology 19, 140–145 (2009). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
