This is a preprint.
Epistasis regulates genetic control of cardiac hypertrophy
- PMID: 38045390
- PMCID: PMC10690313
- DOI: 10.21203/rs.3.rs-3509208/v1
Epistasis regulates genetic control of cardiac hypertrophy
Update in
-
Epistasis regulates genetic control of cardiac hypertrophy.Nat Cardiovasc Res. 2025 Jun;4(6):740-760. doi: 10.1038/s44161-025-00656-8. Epub 2025 Jun 5. Nat Cardiovasc Res. 2025. PMID: 40473955 Free PMC article.
Abstract
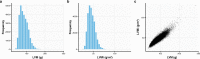
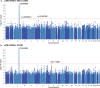
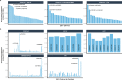
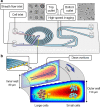
The combinatorial effect of genetic variants is often assumed to be additive. Although genetic variation can clearly interact non-additively, methods to uncover epistatic relationships remain in their infancy. We develop low-signal signed iterative random forests to elucidate the complex genetic architecture of cardiac hypertrophy. We derive deep learning-based estimates of left ventricular mass from the cardiac MRI scans of 29,661 individuals enrolled in the UK Biobank. We report epistatic genetic variation including variants close to CCDC141, IGF1R, TTN, and TNKS. Several loci not prioritized by univariate genome-wide association analysis are identified. Functional genomic and integrative enrichment analyses reveal a complex gene regulatory network in which genes mapped from these loci share biological processes and myogenic regulatory factors. Through a network analysis of transcriptomic data from 313 explanted human hearts, we show that these interactions are preserved at the level of the cardiac transcriptome. We assess causality of epistatic effects via RNA silencing of gene-gene interactions in human induced pluripotent stem cell-derived cardiomyocytes. Finally, single-cell morphology analysis using a novel high-throughput microfluidic system shows that cardiomyocyte hypertrophy is non-additively modifiable by specific pairwise interactions between CCDC141 and both TTN and IGF1R. Our results expand the scope of genetic regulation of cardiac structure to epistasis.
Conflict of interest statement
Competing interests E.A.A. is a Founder of Personalis, Deepcell, Svexa, RCD Co, and Parameter Health; Advisor to Oxford Nanopore, SequenceBio, and Pacific Biosciences; and a non-executive director for AstraZeneca. C.S.W. is a consultant for Tensixteen Bio and Renovacor. V.N.P. is an SAB member for and receives research support from BioMarin, Inc, and is a consultant for Constantiam, Inc. and viz.ai. The remaining authors declare no competing interests.
Figures








References
-
- Weldy C. S. & Ashley E. A. Towards precision medicine in heart failure. Nat. Rev. Cardiol. 1–18 (2021). - PubMed
-
- Sharir T. et al. Ventricular systolic assessment in patients with dilated cardiomyopathy by preload-adjusted maximal power. Validation and noninvasive application. Circulation 89, 2045–2053 (1994). - PubMed
-
- Udelson J. E. 3rd, Bacharach R. O. C., Rumble S. L., T. F. & Bonow R. O. Beta-adrenergic stimulation with isoproterenol enhances left ventricular diastolic performance in hypertrophic cardiomyopathy despite potentiation of myocardial ischemia. Comparison to rapid atrial pacing. Circulation 79, 371–382 (1988). - PubMed
-
- Burkhoff D., Mirsky I. & Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. American Journal of Physiology-Heart and Circulatory Physiology 289, H501–H512 (2005). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

