This is a preprint.
Preventing evolutionary rescue in cancer
- PMID: 38045391
- PMCID: PMC10690287
- DOI: 10.1101/2023.11.22.568336
Preventing evolutionary rescue in cancer
Abstract
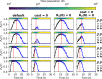
First-line cancer treatment frequently fails due to initially rare therapeutic resistance. An important clinical question is then how to schedule subsequent treatments to maximize the probability of tumour eradication. Here, we provide a theoretical solution to this problem by using mathematical analysis and extensive stochastic simulations within the framework of evolutionary rescue theory to determine how best to exploit the vulnerability of small tumours to stochastic extinction. Whereas standard clinical practice is to wait for evidence of relapse, we confirm a recent hypothesis that the optimal time to switch to a second treatment is when the tumour is close to its minimum size before relapse, when it is likely undetectable. This optimum can lie slightly before or slightly after the nadir, depending on tumour parameters. Given that this exact time point may be difficult to determine in practice, we study windows of high extinction probability that lie around the optimal switching point, showing that switching after the relapse has begun is typically better than switching too early. We further reveal how treatment dose and tumour demographic and evolutionary parameters influence the predicted clinical outcome, and we determine how best to schedule drugs of unequal efficacy. Our work establishes a foundation for further experimental and clinical investigation of this evolutionarily-informed "extinction therapy" strategy.
Keywords: cancer treatment; evolutionary rescue; evolutionary therapy; mathematical oncology; therapeutic resistance.
Figures




References
-
- Pressley Mariyah et al. “Evolutionary Dynamics of Treatment-Induced Resistance in Cancer Informs Understanding of Rapid Evolution in Natural Systems”. In: Frontiers in Ecology and Evolution 9 (2021). ISSN: 2296–701X. URL: https://www.frontiersin.org/articles/10.3389/fevo.2021.681121 (visited on 03/30/2023). - DOI
-
- Greaves Mel and Maley Carlo C.. “Clonal evolution in cancer”. en. In: Nature 481.7381 (Jan. 2012). Number: 7381 Publisher: Nature Publishing Group, pp. 306–313. ISSN: 1476–4687. DOI: 10.1038/nature10762. URL: https://www.nature.com/articles/nature10762 (visited on 09/01/2022). - DOI - PMC - PubMed
-
- Korolev Kirill S., Xavier Joao B., and Gore Jeff. “Turning ecology and evolution against cancer”. en. In: Nature Reviews Cancer 14.5 (May 2014), pp. 371–380. ISSN: 1474–1768. - PubMed
-
- Enriquez-Navas Pedro M., Wojtkowiak Jonathan W., and Gatenby Robert A.. “Application of Evolutionary Principles to Cancer Therapy”. In: Cancer Research 75.22 (Nov. 2015), pp. 4675–4680. ISSN: 0008–5472. DOI: 10.1158/0008-5472.CAN-15-1337. eprint: https://aacrjournals.org/cancerres/article-pdf/75/22/4675/2937393/4675.pdf. URL: 10.1158/0008-5472.CAN-15-1337. - DOI - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
