Usefulness of receptor binding domain protein-based serodiagnosis of COVID-19
- PMID: 38045864
- PMCID: PMC10687696
- DOI: 10.1016/j.ijregi.2023.11.001
Usefulness of receptor binding domain protein-based serodiagnosis of COVID-19
Abstract
Objectives: This study evaluated the performance of recombinant receptor binding domain (RBD) protein-based enzyme-linked immunosorbent assays (RBD-ELISAs) for detecting anti-SARS-CoV-2 immunoglobulin (Ig) G and IgM antibodies.
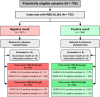
Methods: In this study, 705 sera from SARS-CoV-2-infected individuals and 315 sera from healthy individuals were analyzed.
Results: The RBD-ELISA IgG exhibited high specificity (99.1%) and moderate sensitivity (48.0%), with an overall diagnostic accuracy of 73.5%. RBD-ELISA IgM demonstrated specificity at 94.6% and sensitivity at 51.1%, with an accuracy of 72.8%. Both assays displayed improved performance when analyzing samples collected 15-21 days post-symptom onset, achieving sensitivity and accuracy exceeding 88% and 90%, respectively. Combining RBD-ELISA IgG and IgM in parallel analysis enhanced sensitivity to 98.6% and accuracy to 96.2%. Comparing these RBD-ELISAs with commercially available tests, the study found overlapping sensitivity and similar specificity values. Notably, the combined RBD-ELISA IgG and IgM showed superior performance. Cross-reactivity analysis revealed low false-positive rates (4.4% for IgG, 3.7% for IgM), primarily with viral infections.
Conclusion: This research underscores the potential of RBD-based ELISAs for COVID-19 diagnosis, especially when assessing samples collected 15-21 days post-symptom onset and utilizing a parallel testing approach. The RBD protein's immunogenicity and specificity make it a valuable tool for serodiagnosis, offering an alternative to polymerase chain reaction-based methods, particularly in resource-limited settings.
Keywords: Antibody detection; Diagnostic performance; Recombinant RBD protein; SARS-CoV-2; Serodiagnosis.
© 2023 The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

