Genetic Patterns of Selected Muscular Dystrophies in the Muscular Dystrophy Surveillance, Tracking, and Research Network
- PMID: 38045992
- PMCID: PMC10692796
- DOI: 10.1212/NXG.0000000000200113
Genetic Patterns of Selected Muscular Dystrophies in the Muscular Dystrophy Surveillance, Tracking, and Research Network
Abstract
Background and objectives: To report the genetic etiologies of Emery-Dreifuss muscular dystrophy (EDMD), limb-girdle muscular dystrophy (LGMD), congenital muscular dystrophy (CMD), and distal muscular dystrophy (DD) in 6 geographically defined areas of the United States.
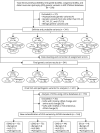
Methods: This was a cross-sectional, population-based study in which we studied the genes and variants associated with muscular dystrophy in individuals who were diagnosed with and received care for EDMD, LGMD, CMD, and DD from January 1, 2008, through December 31, 2016, in the 6 areas of the United States covered by the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet). Variants of unknown significance (VUSs) from the original genetic test reports were reanalyzed for changes in interpretation.
Results: Among 243 individuals with definite or probable muscular dystrophy, LGMD was the most common diagnosis (138 cases), followed by CMD (62 cases), DD (22 cases), and EDMD (21 cases). There was a higher proportion of male individuals compared with female individuals, which persisted after excluding X-linked genes (EMD) and autosomal genes reported to have skewed gender ratios (ANO5, CAV3, and LMNA). The most common associated genes were FKRP, CAPN3, ANO5, and DYSF. Reanalysis yielded more definitive variant interpretations for 60 of 144 VUSs, with a mean interval between the original clinical genetic test of 8.11 years for all 144 VUSs and 8.62 years for the 60 reclassified variants. Ten individuals were found to have monoallelic pathogenic variants in genes known to be primarily recessive.
Discussion: This study is distinct for being an examination of 4 types of muscular dystrophies in selected geographic areas of the United States. The striking proportion of resolved VUSs demonstrates the value of periodic re-examinations of these variants. Such re-examinations will resolve some genetic diagnostic ambiguities before initiating repeat testing or more invasive diagnostic procedures such as muscle biopsy. The presence of monoallelic pathogenic variants in recessive genes in our cohort indicates that some individuals with muscular dystrophy continue to face incomplete genetic diagnoses; further refinements in genetic knowledge and diagnostic approaches will optimize diagnostic information for these individuals.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
P.B. Kang has served on advisory boards for Sarepta Therapeutics, NS Pharma, and Teneofour; and has served as a consultant for Novartis and Neurogene. M. Jorand-Fletcher reports no disclosures. W. Zhang reports no disclosures. S.W. McDermott reports no disclosures. R. Berry reports no disclosures. C. Chambers reports no disclosures. K.N. Wong reports no disclosures. Y. Mohamed reports no disclosures. S. Thomas reports no disclosures. Y.S. Venkatesh reports no disclosures. C. Westfield reports no disclosures. N. Whitehead reports no disclosures. N.E. Johnson has received grant funding from the NIH (R01 NS104010 and R21 TR003184), CDC (U01 DD001242), and the FDA (R01 FD006071). He receives research funds from Dyne, AveXis, Vertex Pharmaceuticals, Fulcrum Therapeutics, ML Bio, Sarepta, Triplet Therapeutics, Avidity Biosciences, and AMO Pharma. He has provided consultation for AMO Pharma, AveXis, Fulcrum Therapeutics, Dyne, Avidity, Vertex, and Entrada. He receives licensing fees from the University of Rochester for the CCMDHI and CMTHI. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NG.
Figures
References
-
- Polavarapu K, Mathur A, Joshi A, et al. . A founder mutation in the GMPPB gene [c.1000G > A (p.Asp334Asn)] causes a mild form of limb-girdle muscular dystrophy/congenital myasthenic syndrome (LGMD/CMS) in South Indian patients. Neurogenetics. 2021;22(4):271-285. doi:10.1007/s10048-021-00658-1 - DOI - PubMed
-
- Pantoja-Melendez CA, Miranda-Duarte A, Roque-Ramirez B, Zenteno JC. Epidemiological and molecular characterization of a mexican population isolate with high prevalence of limb-girdle muscular dystrophy type 2A due to a novel calpain-3 mutation. PLoS One. 2017;12(1):e0170280. doi:10.1371/journal.pone.0170280 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous