Phase 2 study of upacicalcet in Japanese haemodialysis patients with secondary hyperparathyroidism: an intraindividual dose-adjustment study
- PMID: 38045997
- PMCID: PMC10689153
- DOI: 10.1093/ckj/sfad213
Phase 2 study of upacicalcet in Japanese haemodialysis patients with secondary hyperparathyroidism: an intraindividual dose-adjustment study
Abstract
Background: Upacicalcet is a novel small-molecule calcimimetic agent developed for intravenous injection. Here, we evaluated the long-term efficacy and safety of upacicalcet treatment via intraindividual dose adjustment in haemodialysis patients with secondary hyperparathyroidism (SHPT).
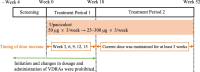
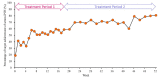
Methods: A phase 2, multicentre, open-label, single-arm study was conducted. Upacicalcet was administered for 52 weeks; the starting dose was 50 μg thrice a week, and then adjusted to 25, 50, 100, 150, 200, 250, or 300 μg, according to the dose-adjustment method set in the protocol. The primary endpoint was the percentage of patients with serum intact parathyroid hormone (iPTH) level achieving a target range of 60-240 pg/mL (target achievement rate) at week 18.
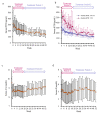
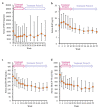
Results: A total of 58 patients were administered upacicalcet. The target achievement rate of serum iPTH level at week 18 was 57.9%, which increased to 80.8% at week 52. The serum-corrected calcium (cCa) level decreased immediately after upacicalcet administration, but no further decrease was observed. Adverse events were observed in 94.8% of patients, and adverse drug reactions (ADRs) occurred in 20.7% of patients. The most common ADR was decreased adjusted calcium in eight patients; dizziness occurred as a serious ADR in one patient. The serum cCa level of patients who interrupted upacicalcet treatment at a serum cCa level of <7.5 mg/dL recovered to ≥7.5 mg/dL immediately after the interruption.
Conclusions: In haemodialysis patients with SHPT, upacicalcet doses of 25-300 μg for 52 weeks were found to be highly effective and well-tolerated, with minor safety concerns.
Keywords: calcimimetics; haemodialysis patients; parathyroid hormone; secondary hyperparathyroidism; upacicalcet.
© The Author(s) 2023. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
D.I. has received personal fees from SKK, Kyowa Kirin, Kissei Pharmaceutical, and Ono Pharmaceutical. F.K. has received personal fees from SKK, Kyowa Kirin, and Ono Pharmaceutical. M.T. has received personal fees from SKK, Kyowa Kirin, AstraZeneca, Torii Pharmaceutical, Astellas Pharma, Kissei Pharmaceutical, Chugai Pharmaceutical, Mitsubishi Tanabe Pharma Corporation, Kowa, Bayer Yakuhin. M.F. has received personal fees from SKK, Kyowa Kirin, Bayer Japan, Kissei Pharmaceutical, Torii Pharmaceutical, Ono Pharmaceutical, and Astra Zeneca; grants from Kyowa Kirin and Ono Pharmaceutical. S.Y., H.Y., K.A., K.H., Y.I., and D.H. are employees of SKK. T.A. has received personal fees from SKK, Kyowa Kirin, Bayer, Astellas Pharma, GlaxoSmithKline, Japan Tobacco, Nipro, Torii Pharmaceutical, Kissei Pharmaceutical, Chugai Pharmaceutical, Mitsubishi Tanabe Pharma, Ono Pharmaceutical, and Fuso Pharmaceutical Industries.
Figures




Similar articles
-
Long-Term Efficacy and Safety of Upacicalcet in Japanese Hemodialysis Patients with Secondary Hyperparathyroidism: Open-Label 52-Week Study.Am J Nephrol. 2025;56(1):70-84. doi: 10.1159/000541493. Epub 2024 Sep 19. Am J Nephrol. 2025. PMID: 39299219 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Upacicalcet in Hemodialysis Patients with Secondary Hyperparathyroidism: A Randomized Placebo-Controlled Trial.Clin J Am Soc Nephrol. 2023 Oct 1;18(10):1300-1309. doi: 10.2215/CJN.0000000000000253. Epub 2023 Sep 11. Clin J Am Soc Nephrol. 2023. PMID: 37696667 Free PMC article. Clinical Trial.
-
Pharmacological profile of upacicalcet, a novel positive allosteric modulator of calcium-sensing receptor, in vitro and in vivo.Eur J Pharmacol. 2023 Oct 5;956:175936. doi: 10.1016/j.ejphar.2023.175936. Epub 2023 Aug 2. Eur J Pharmacol. 2023. PMID: 37541363
-
Upacicalcet: First Approval.Drugs. 2021 Sep;81(13):1593-1596. doi: 10.1007/s40265-021-01578-y. Drugs. 2021. PMID: 34390486 Review.
-
Intermittent oral 1alpha-hydroxyvitamin D2 is effective and safe for the suppression of secondary hyperparathyroidism in haemodialysis patients. 1alphaD2 Study Group.Nephrol Dial Transplant. 1998;13 Suppl 3:68-72. doi: 10.1093/ndt/13.suppl_3.68. Nephrol Dial Transplant. 1998. PMID: 9568825 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

