"Click Chemistry": An Emerging Tool for Developing a New Class of Structural Motifs against Various Neurodegenerative Disorders
- PMID: 38046293
- PMCID: PMC10688180
- DOI: 10.1021/acsomega.3c04960
"Click Chemistry": An Emerging Tool for Developing a New Class of Structural Motifs against Various Neurodegenerative Disorders
Abstract
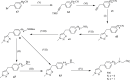
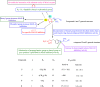
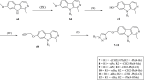
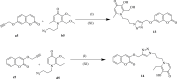
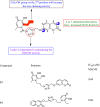
Click chemistry is a set of easy, atom-economical reactions that are often utilized to combine two desired chemical entities. Click chemistry accelerates lead identification and optimization, reduces the complexity of chemical synthesis, and delivers extremely high yields without undesirable byproducts. The most well-known click chemistry reaction is the 1,3-dipolar cycloaddition of azides and alkynes to form 1,2,3-triazoles. The resulting 1,2,3-triazoles can serve as both bioisosteres and linkers, leading to an increase in their use in the field of drug discovery. The current Review focuses on the use of click chemistry to identify new molecules for treating neurodegenerative diseases and in other areas such as peptide targeting and the quantification of biomolecules.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

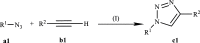



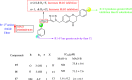
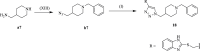
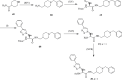

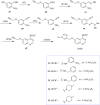
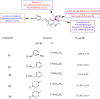




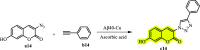
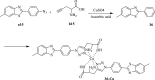
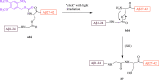
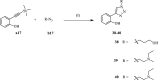
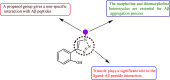

References
-
- Chaturvedi P.; Chaturvedi N.; Gupta S.; Mishra A.; Singh M.; Siddhartha T. Click Chemistry: A New Approach for Drug Discovery. Int. J. Pharm. Sci. Rev. Res. 2011, 10, 111–117.
-
- The Editors of Encyclopaedia Britannica . K. Barry Sharpless. In Encyclopaedia Britannica; Encyclopaedia Britannica, Inc.: 2023.
-
- Rostovtsev V. V; Green L. G.; Fokin V. V.; Sharpless K. B.; Coolen H. K.; van Leeuwen P. W.; Nolte R. J.; Harrowfield J. M. In Calixarenes; Kluwer Academic Publishers: 2002; Vol. 114.
Publication types
LinkOut - more resources
Full Text Sources

