Modeling microbiota-associated human diseases: from minimal models to complex systems
- PMID: 38046357
- PMCID: PMC10688821
- DOI: 10.20517/mrr.2022.01
Modeling microbiota-associated human diseases: from minimal models to complex systems
Abstract
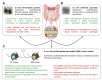
Alterations in the intestinal microbiota are associated with various human diseases of the digestive system, including obesity and its associated metabolic diseases, inflammatory bowel diseases (IBD), and colorectal cancer (CRC). All three diseases are characterized by modifications of the richness, composition, and metabolic functions of the human intestinal microbiota. Despite being multi-factorial diseases, studies in germ-free animal models have unarguably identified the intestinal microbiota as a causal driver of disease pathogenesis. However, for an increased mechanistic understanding of microbial signatures in human diseases, models require detailed refinement to closely mimic the human microbiota and reflect the complexity and range of dysbiosis observed in patients. The transplantation of human fecal microbiota into animal models represents a powerful tool for studying the causal and functional role of the dysbiotic human microbiome in a pathological context. While human microbiota-associated models were initially employed to study obesity, an increasing number of studies have applied this approach in the context of IBD and CRC over the past decade. In this review, we discuss different approaches that allow the functional validation of the bacterial contribution to human diseases, with emphasis on obesity and its associated metabolic diseases, IBD, and CRC. We discuss the utility of simple models, such as in vitro fermentation systems of the human microbiota and ex vivo intestinal organoids, as well as more complex whole organism models. Our focus here lies on human microbiota-associated mouse models in the context of all three diseases, as well as highlighting the advantages and limitations of this approach.
Keywords: Human microbiota-associated mouse models; colorectal cancer; fecal microbiota transplantation; host-microbiota interactions; inflammatory bowel diseases; metabolic diseases; obesity.
© The Author(s) 2022.
Conflict of interest statement
All authors declared that there are no conflicts of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
