A human mesenchymal spheroid prototype to replace moderate severity animal procedures in leukaemia drug testing
- PMID: 38046539
- PMCID: PMC10691310
- DOI: 10.12688/f1000research.123084.2
A human mesenchymal spheroid prototype to replace moderate severity animal procedures in leukaemia drug testing
Abstract
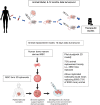
Patient derived xenograft (PDX) models are regarded as gold standard preclinical models in leukaemia research, especially in testing new drug combinations where typically 45-50 mice are used per assay. 9000 animal experiments are performed annually in the UK in leukaemia research with these expensive procedures being classed as moderate severity, meaning they cause significant pain, suffering and visible distress to animal's state. Furthermore, not all clinical leukaemia samples engraft and when they do data turnaround time can be between 6-12 months. Heavy dependence on animal models is because clinical leukaemia samples do not proliferate in vitro. Alternative cell line models though popular for drug testing are not biomimetic - they are not dependent on the microenvironment for survival, growth and treatment response and being derived from relapse samples they do not capture the molecular complexity observed at disease presentation. Here we have developed an in vitro platform to rapidly establish co-cultures of patient-derived leukaemia cells with 3D bone marrow mesenchyme spheroids, BM-MSC-spheroids. We optimise protocols for developing MSC-spheroid leukaemia co-culture using clinical samples and deliver drug response data within a week. Using three patient samples representing distinct cytogenetics we show that patient-derived-leukaemia cells show enhanced proliferation when co-cultured with MSC-spheroids. In addition, MSC-spheroids provided improved protection against treatment. This makes our spheroids suitable to model treatment resistance - a major hurdle in current day cancer management Given this 3Rs approach is 12 months faster (in delivering clinical data), is a human cell-based biomimetic model and uses 45-50 fewer animals/drug-response assay the anticipated target end-users would include academia and pharmaceutical industry. This animal replacement prototype would facilitate clinically translatable research to be performed with greater ethical, social and financial sustainability.
Keywords: 3D models; Animal replacement; Preclinical models; cancer research; leukaemia.
Copyright: © 2023 Wilson A et al.
Conflict of interest statement
No competing interests were disclosed.
Figures




References
-
- Seyhan AA: Lost in translation: the valley of death across preclinical and clinical divide – identification of problems and overcoming obstacles. Transl Med Commun. 2019;4:18. 10.1186/s41231-019-0050-7 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

