Comparison of Shigella GMMA and glycoconjugate four-component formulations in animals
- PMID: 38046812
- PMCID: PMC10690372
- DOI: 10.3389/fmolb.2023.1284515
Comparison of Shigella GMMA and glycoconjugate four-component formulations in animals
Abstract
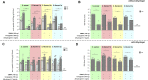
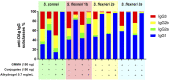
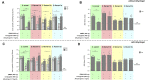
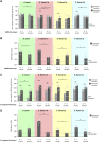
Shigellosis is leading bacterial cause of diarrhea with high prevalence in children younger than 5 years in low- and middle-income countries, and increasing number of reports of Shigella cases associated to anti-microbial resistance. No vaccines against Shigella are still licensed, but different candidates based on the O-antigen portion of lipopolysaccharides are in clinic. Generalized Modules for Membrane Antigens (GMMA) have been proposed as an alternative delivery system for the O-antigen, and a 4-component vaccine candidate (altSonflex1-2-3), containing GMMA from S. sonnei and S. flexneri 1b, 2a and 3a is being tested in a phase 1/2 clinical trial, with the aim to elicit broad protection against the most prevalent Shigella serotypes. Here, the 4-component GMMA vaccine candidate has been compared to a more traditional glycoconjugate formulation for the ability to induce functional antibodies in mice and rabbits. In mice, in the absence of Alhydrogel, GMMA induce higher IgG antibodies than glycoconjugates and stronger bactericidal titers against all Shigella serotypes. In the presence of Alhydrogel, GMMA induce O-antigen specific IgG levels similar to traditional glycoconjugates, but with a broader range of IgG subclasses, resulting in stronger bactericidal activity. In rabbits, GMMA elicit higher functional antibodies than glycoconjugates against S. sonnei, and similar responses to S. flexneri 1b, 2a and 3a, independently from the presence of Alhydrogel. Different O-antigen based vaccines against Shigella are now in clinical stage and it will be of particular interest to understand how the preclinical findings in the different animal models translate in humans.
Keywords: GMMA; O-antigen; Shigella; glycoconjugate; multicomponent; vaccine.
Copyright © 2023 Di Benedetto, Mancini, Caradonna, Aruta, Giannelli, Rossi and Micoli.
Conflict of interest statement
This work was undertaken at the request of and sponsored by GlaxoSmithKline Biologicals SA. GSK Vaccines Institute for Global Health Srl is an affiliate of GlaxoSmithKline Biologicals SA. All authors are employees of the GSK group of companies. RD, OR, and FMi own GSK shares. RD also participates in a PhD program at GSK.
Figures

References
-
- Angela B., Stefano R., Daniela P. (2005). Separation of unconjugated and conjugated saccharide by solid phase extraction. EP20050718410.
LinkOut - more resources
Full Text Sources

