Thermal Oscillations of Nanobubbles
- PMID: 38047571
- PMCID: PMC10722608
- DOI: 10.1021/acs.nanolett.3c03052
Thermal Oscillations of Nanobubbles
Abstract
Nanobubble cavitation is advancing technologies in enhanced wastewater treatment, cancer therapy and diagnosis, and microfluidic cleaning. Current macroscale models predict that nanobubble oscillations should be isothermal, yet recent studies suggest that they are adiabatic with an associated increase in natural frequency, which becomes challenging when characterizing nanobubble sizes using ultrasound in experiments. We derive a new theoretical model that considers the nonideal nature of the nanobubble's internal gas phase and nonequilibrium effects, by employing the van der Waals (vdW) equation of state and implementing a temperature jump term at the liquid-gas interface, respectively, finding excellent agreement with molecular dynamics (MD) simulations. Our results reveal how adiabatic behavior could be erroneously interpreted when analyzing the thermal response of the gas using the commonly employed polytropic process and explain instead how nanobubble oscillations are physically closer to their isothermal limit.
Keywords: cavitation; nanobubbles; nonequilibrium gas; nonideal gas; oscillations; thermodynamics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

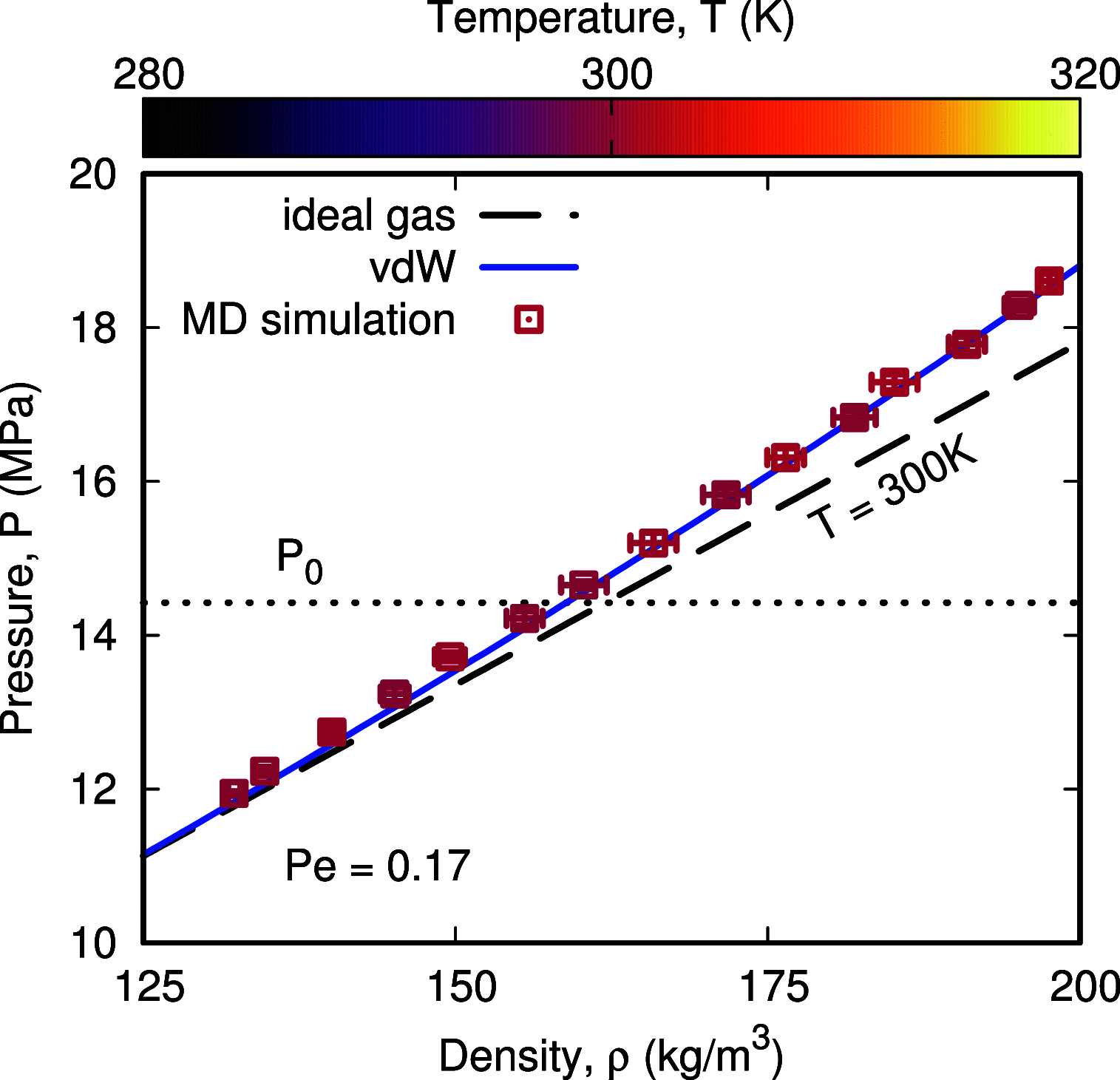
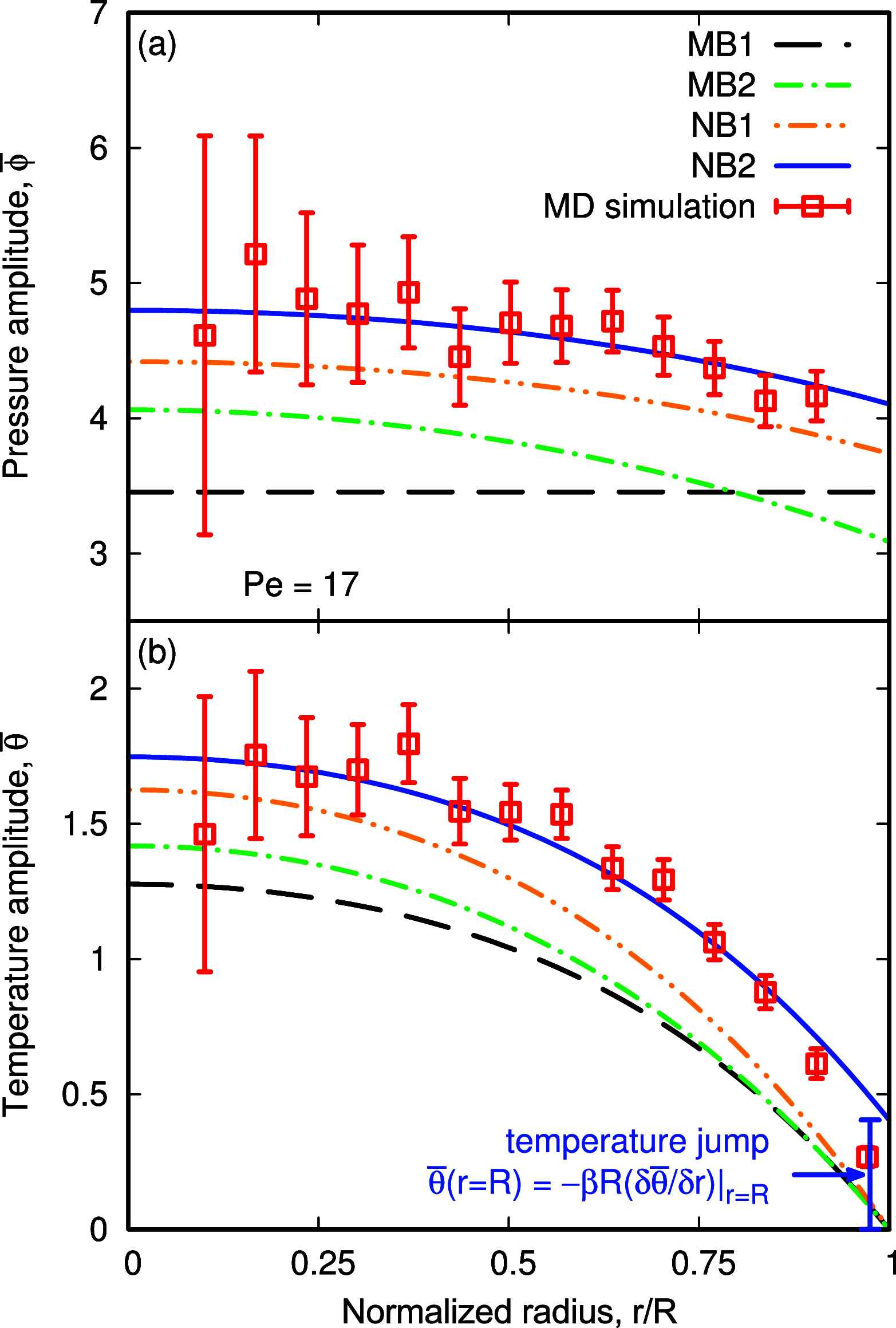
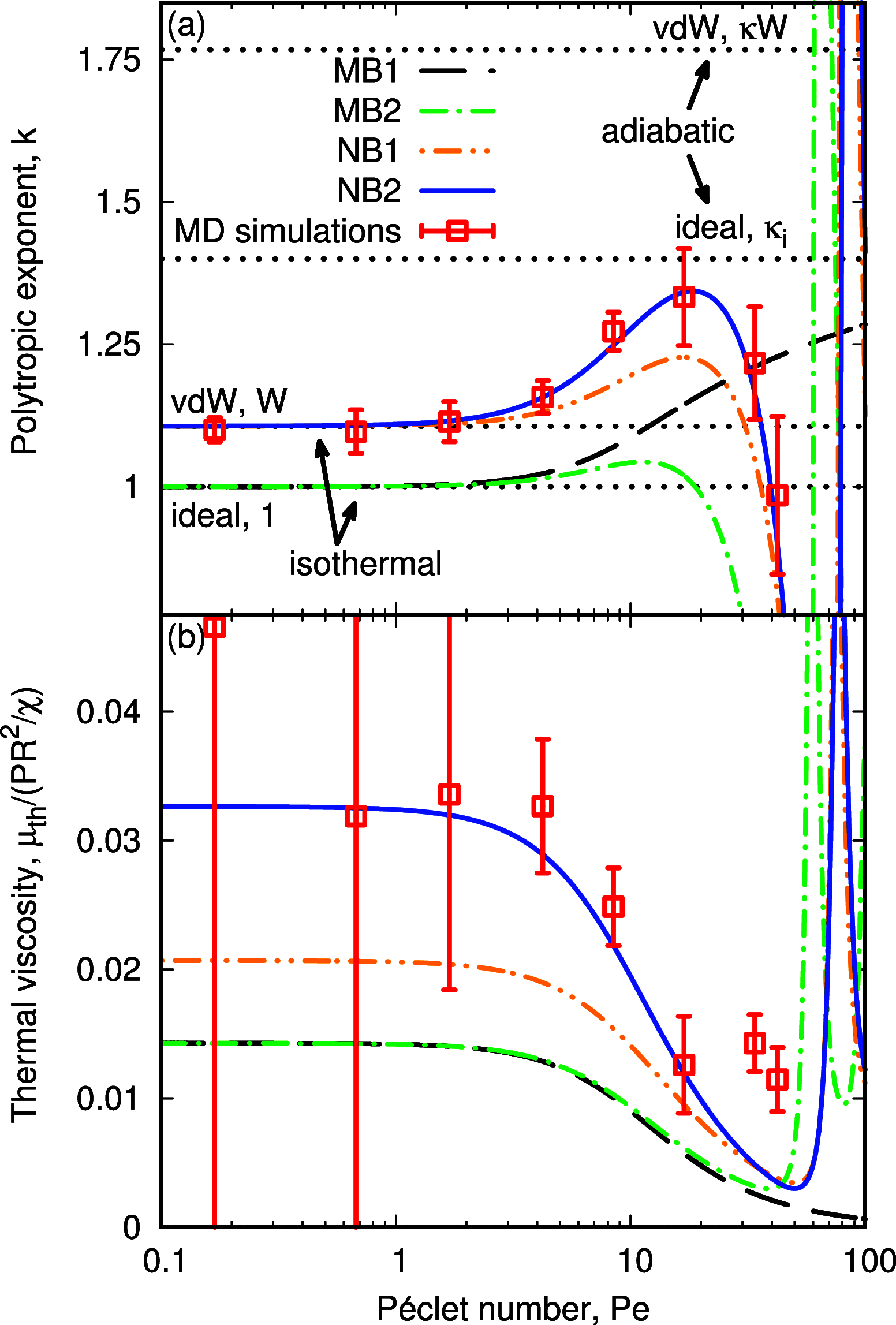

References
-
- Brennen C. E.Cavitation and Bubble Dynamics; Cambridge University Press: 2013.
-
- Brems S.; Hauptmann M.; Camerotto E.; Pacco A.; Kim T.-G.; Xu X.; Wostyn K.; Mertens P.; De Gendt S. Nanoparticle Removal with Megasonics: A Review. ECS J. Solid State Sci. Technol. 2014, 3, N3010–N3015. 10.1149/2.004401jss. - DOI
LinkOut - more resources
Full Text Sources

