Remifentanil-induced hyperalgesia in healthy volunteers: a systematic review and meta-analysis of randomized controlled trials
- PMID: 38047761
- PMCID: PMC11017745
- DOI: 10.1097/j.pain.0000000000003119
Remifentanil-induced hyperalgesia in healthy volunteers: a systematic review and meta-analysis of randomized controlled trials
Abstract
Recent literature suggests that the withdrawal of remifentanil (RF) infusion can be associated with hyperalgesia in clinical and nonclinical settings. We performed a systematic review and a meta-analysis of randomized controlled trials with cross-over design, to assess the effect of discontinuing RF infusion on pain intensity and areas of hyperalgesia and allodynia in healthy volunteers. Nine studies were included. The intervention treatment consisted in RF infusion that was compared with placebo (saline solution). The primary outcome was pain intensity assessment at 30 ± 15 minutes after RF or placebo discontinuation, assessed by any pain scale and using any quantitative sensory testing. Moreover, postwithdrawal pain scores were compared with baseline scores in each treatment. Secondary outcomes included the areas (% of basal values) of hyperalgesia and allodynia. Subjects during RF treatment reported higher pain scores after discontinuation than during treatment with placebo [standardized mean difference (SMD): 0.50, 95% confidence interval (CI): 0.03-0.97; P = 0.04, I 2 = 71%]. A significant decrease in pain scores, compared with baseline values, was found in the placebo treatment (SMD: -0.87, 95% CI: -1.61 to -0.13; P = 0.02, I 2 = 87%), but not in the RF treatment (SMD: -0.28, 95% CI: -1.18 to 0.62; P = 0.54, I 2 = 91%). The area of hyperalgesia was larger after RF withdrawal (SMD: 0.55; 95% CI: 0.27-0.84; P = 0.001; I 2 = 0%). The area of allodynia did not vary between treatments. These findings suggest that the withdrawal of RF induces a mild but nonclinically relevant degree of hyperalgesia in HVs, likely linked to a reduced pain threshold.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
V.D.F. is an employee of Angelini Pharma since April 2023. The other authors declare no conflict of interest.
Figures
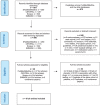

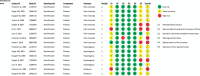

References
-
- Aceto P, Dello Russo C, Lai C, Perilli V, Fucci N, De Giovanni N, Piras A, Navarra P, Sollazzi L. Relationship between blood remifentanil concentration and stress hormone levels during pneumoperitoneum in patients undergoing laparoscopic cholecystectomy. Eur Rev Med Pharmacol Sci 2017;21:4419–22. - PubMed
-
- Aceto P, Dello Russo C, Punzo G, Luca E, Crea MA, Modesti C, Sacco T, De Cicco R, Tosi A, Ceaichisciuc I, Marusco I, Schipa C, D'Angelo F, Perilli V, Sollazzi L. Success of fast-track anesthesia. In: Bernhardt LV, editor. Advances in medicine and biology. Hauppauge, NY: Nova Science Publishers, 2019. p. 88–122.
-
- Aceto P, Lai C, Perilli V, Sacco T, Modesti C, Raffaelli M, Sollazzi L. Factors affecting acute pain perception and analgesics consumption in patients undergoing bariatric surgery. Physiol Behav 2016;163:1–6. - PubMed
-
- Adams TJ, Aljohani DM, Forget P. Perioperative opioids: a narrative review contextualising new avenues to improve prescribing. Br J Anaesth 2023;130:709–18. - PubMed
-
- Aguado D, Abreu M, Benito J, García-Fernández J, Gómez de Segura IA. Ketamine and remifentanil interactions on the sevoflurane minimum alveolar concentration and acute opioid tolerance in the rat. Anesth Analg 2011;113:505–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

