Intravenous Levothyroxine for Unstable Brain-Dead Heart Donors
- PMID: 38048188
- PMCID: PMC10752368
- DOI: 10.1056/NEJMoa2305969
Intravenous Levothyroxine for Unstable Brain-Dead Heart Donors
Abstract
Background: Hemodynamic instability and myocardial dysfunction are major factors preventing the transplantation of hearts from organ donors after brain death. Intravenous levothyroxine is widely used in donor care, on the basis of observational data suggesting that more organs may be transplanted from donors who receive hormonal supplementation.
Methods: In this trial involving 15 organ-procurement organizations in the United States, we randomly assigned hemodynamically unstable potential heart donors within 24 hours after declaration of death according to neurologic criteria to open-label infusion of intravenous levothyroxine (30 μg per hour for a minimum of 12 hours) or saline placebo. The primary outcome was transplantation of the donor heart; graft survival at 30 days after transplantation was a prespecified recipient safety outcome. Secondary outcomes included weaning from vasopressor therapy, donor ejection fraction, and number of organs transplanted per donor.
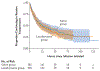
Results: Of the 852 brain-dead donors who underwent randomization, 838 were included in the primary analysis: 419 in the levothyroxine group and 419 in the saline group. Hearts were transplanted from 230 donors (54.9%) in the levothyroxine group and 223 (53.2%) in the saline group (adjusted risk ratio, 1.01; 95% confidence interval [CI], 0.97 to 1.07; P = 0.57). Graft survival at 30 days occurred in 224 hearts (97.4%) transplanted from donors assigned to receive levothyroxine and 213 hearts (95.5%) transplanted from donors assigned to receive saline (difference, 1.9 percentage points; 95% CI, -2.3 to 6.0; P<0.001 for noninferiority at a margin of 6 percentage points). There were no substantial between-group differences in weaning from vasopressor therapy, ejection fraction on echocardiography, or organs transplanted per donor, but more cases of severe hypertension and tachycardia occurred in the levothyroxine group than in the saline group.
Conclusions: In hemodynamically unstable brain-dead potential heart donors, intravenous levothyroxine infusion did not result in significantly more hearts being transplanted than saline infusion. (Funded by Mid-America Transplant and others; ClinicalTrials.gov number, NCT04415658.).
Copyright © 2023 Massachusetts Medical Society.
Figures
Comment in
-
Levothyroxine supplementation does not improve heart transplantation from brain-dead donors.Nat Rev Cardiol. 2024 Feb;21(2):74. doi: 10.1038/s41569-023-00980-1. Nat Rev Cardiol. 2024. PMID: 38066088 No abstract available.
-
Intravenous Levothyroxine for Unstable Brain-Dead Heart Donors.N Engl J Med. 2024 Feb 8;390(6):574-575. doi: 10.1056/NEJMc2314959. N Engl J Med. 2024. PMID: 38324494 No abstract available.
-
Intravenous Levothyroxine for Unstable Brain-Dead Heart Donors.N Engl J Med. 2024 Feb 8;390(6):575. doi: 10.1056/NEJMc2314959. N Engl J Med. 2024. PMID: 38324495 No abstract available.
-
Intravenous Levothyroxine for Unstable Brain-Dead Heart Donors. Reply.N Engl J Med. 2024 Feb 8;390(6):575-576. doi: 10.1056/NEJMc2314959. N Engl J Med. 2024. PMID: 38324496 No abstract available.
References
-
- Smith M. Physiologic changes during brain stem death — lessons for management of the organ donor. J Heart Lung Transplant 2004;23:Suppl:S217–S222. - PubMed
-
- Novitzky D, Cooper DK, Morrell D, Isaacs S. Change from aerobic to anaerobic metabolism after brain death, and reversal following triiodothyronine therapy. Transplantation 1988;45:32–6. - PubMed
-
- Novitzky D, Cooper DKC, Rosendale JD, Kauffman HM. Hormonal therapy of the brain-dead organ donor: experimental and clinical studies. Transplantation 2006;82:1396–401. - PubMed
-
- Rosendale JD, Kauffman HM, McBride MA, et al. Hormonal resuscitation yields more transplanted hearts, with improved early function. Transplantation 2003;75:1336–41. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
