Deletion of IFT20 exclusively in the RPE ablates primary cilia and leads to retinal degeneration
- PMID: 38048369
- PMCID: PMC10721183
- DOI: 10.1371/journal.pbio.3002402
Deletion of IFT20 exclusively in the RPE ablates primary cilia and leads to retinal degeneration
Abstract
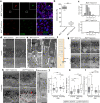
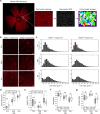
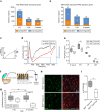
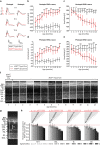
Vision impairment places a serious burden on the aging society, affecting the lives of millions of people. Many retinal diseases are of genetic origin, of which over 50% are due to mutations in cilia-associated genes. Most research on retinal degeneration has focused on the ciliated photoreceptor cells of the retina. However, the contribution of primary cilia in other ocular cell types has largely been ignored. The retinal pigment epithelium (RPE) is a monolayer epithelium at the back of the eye intricately associated with photoreceptors and essential for visual function. It is already known that primary cilia in the RPE are critical for its development and maturation; however, it remains unclear whether this affects RPE function and retinal tissue homeostasis. We generated a conditional knockout mouse model, in which IFT20 is exclusively deleted in the RPE, ablating primary cilia. This leads to defective RPE function, followed by photoreceptor degeneration and, ultimately, vision impairment. Transcriptomic analysis offers insights into mechanisms underlying pathogenic changes, which include transcripts related to epithelial homeostasis, the visual cycle, and phagocytosis. Due to the loss of cilia exclusively in the RPE, this mouse model enables us to tease out the functional role of RPE cilia and their contribution to retinal degeneration, providing a powerful tool for basic and translational research in syndromic and non-syndromic retinal degeneration. Non-ciliary mechanisms of IFT20 in the RPE may also contribute to pathogenesis and cannot be excluded, especially considering the increasing evidence of non-ciliary functions of ciliary proteins.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

