'Trapped re-entry' as source of acute focal atrial arrhythmias
- PMID: 38048392
- PMCID: PMC10939464
- DOI: 10.1093/cvr/cvad179
'Trapped re-entry' as source of acute focal atrial arrhythmias
Abstract
Aims: Diseased atria are characterized by functional and structural heterogeneities, adding to abnormal impulse generation and propagation. These heterogeneities are thought to lie at the origin of fractionated electrograms recorded during sinus rhythm (SR) in atrial fibrillation (AF) patients and are assumed to be involved in the onset and perpetuation (e.g. by re-entry) of this disorder. The underlying mechanisms, however, remain incompletely understood. Here, we tested whether regions of dense fibrosis could create an electrically isolated conduction pathway (EICP) in which re-entry could be established via ectopy and local block to become 'trapped'. We also investigated whether this could generate local fractionated electrograms and whether the re-entrant wave could 'escape' and cause a global tachyarrhythmia due to dynamic changes at a connecting isthmus.
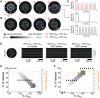
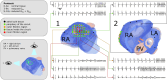
Methods and results: To precisely control and explore the geometrical properties of EICPs, we used light-gated depolarizing ion channels and patterned illumination for creating specific non-conducting regions in silico and in vitro. Insight from these studies was used for complementary investigations in virtual human atria with localized fibrosis. We demonstrated that a re-entrant tachyarrhythmia can exist locally within an EICP with SR prevailing in the surrounding tissue and identified conditions under which re-entry could escape from the EICP, thereby converting a local latent arrhythmic source into an active driver with global impact on the heart. In a realistic three-dimensional model of human atria, unipolar epicardial pseudo-electrograms showed fractionation at the site of 'trapped re-entry' in coexistence with regular SR electrograms elsewhere in the atria. Upon escape of the re-entrant wave, acute arrhythmia onset was observed.
Conclusions: Trapped re-entry as a latent source of arrhythmogenesis can explain the sudden onset of focal arrhythmias, which are able to transgress into AF. Our study might help to improve the effectiveness of ablation of aberrant cardiac electrical signals in clinical practice.
Keywords: Atrial arrhythmias; Cell culture; Computer modelling; Fibrosis; Optogenetics.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures






Comment in
-
Latent drivers for atrial fibrillation and specific patterns of localized fibrosis.Cardiovasc Res. 2024 Mar 14;120(3):215-216. doi: 10.1093/cvr/cvae032. Cardiovasc Res. 2024. PMID: 38376986 No abstract available.
References
-
- Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ 2016;354:i4482. - PubMed
-
- Spach MS, Dolber PC. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res 1986;58(3):356–371. - PubMed
-
- Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections; a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol 1997;20(2):397–413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

