Impaired O2 unloading from stored blood results in diffusion-limited O2 release at tissues: evidence from human kidneys
- PMID: 38048591
- PMCID: PMC10900257
- DOI: 10.1182/blood.2023022385
Impaired O2 unloading from stored blood results in diffusion-limited O2 release at tissues: evidence from human kidneys
Abstract
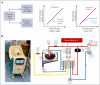
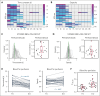
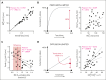
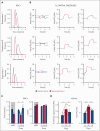
The volume of oxygen drawn from systemic capillaries down a partial pressure gradient is determined by the oxygen content of red blood cells (RBCs) and their oxygen-unloading kinetics, although the latter is assumed to be rapid and, therefore, not a meaningful factor. Under this paradigm, oxygen transfer to tissues is perfusion-limited. Consequently, clinical treatments to optimize oxygen delivery aim at improving blood flow and arterial oxygen content, rather than RBC oxygen handling. Although the oxygen-carrying capacity of blood is increased with transfusion, studies have shown that stored blood undergoes kinetic attrition of oxygen release, which may compromise overall oxygen delivery to tissues by causing transport to become diffusion-limited. We sought evidence for diffusion-limited oxygen release in viable human kidneys, normothermically perfused with stored blood. In a cohort of kidneys that went on to be transplanted, renal respiration correlated inversely with the time-constant of oxygen unloading from RBCs used for perfusion. Furthermore, the renal respiratory rate did not correlate with arterial O2 delivery unless this factored the rate of oxygen-release from RBCs, as expected from diffusion-limited transport. To test for a rescue effect, perfusion of kidneys deemed unsuitable for transplantation was alternated between stored and rejuvenated RBCs of the same donation. This experiment controlled oxygen-unloading, without intervening ischemia, holding all non-RBC parameters constant. Rejuvenated oxygen-unloading kinetics improved the kidney's oxygen diffusion capacity and increased cortical oxygen partial pressure by 60%. Thus, oxygen delivery to tissues can become diffusion-limited during perfusion with stored blood, which has implications in scenarios, such as ex vivo organ perfusion, major hemorrhage, and pediatric transfusion. This trial was registered at www.clinicaltrials.gov as #ISRCTN13292277.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: P.F. and C.C. are founders, shareholders, and directors of OrganOx Ltd, and serve, respectively, as its Chief Medical and Technical Officers for which they receive consultancy fees. R.D., S.K., and J.F. have received consultancy fees from OrganOx Ltd. The remaining authors declare no competing financial interests.
Figures



Comment in
-
Diffusion-limited oxygen delivery.Blood. 2024 Feb 22;143(8):659-660. doi: 10.1182/blood.2023023201. Blood. 2024. PMID: 38386428 No abstract available.
References
-
- Boron WF. In: Medical Physiology. Boulpaep EL, Boron WF, editors. Saunders Elsevier; 2009. Chapter 30: “Ventilation and perfusion of the lungs”; pp. 690–692.
-
- Kobayashi H, Pelster B, Piiper J, Scheid P. Diffusion and perfusion limitation in alveolar O2 exchange: shape of the blood O2 equilibrium curve. Respir Physiol. 1991;83(1):23–34. - PubMed
-
- Piiper J, Scheid P. Model for capillary-alveolar equilibration with special reference to O2 uptake in hypoxia. Respir Physiol. 1981;46(3):193–208. - PubMed
-
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

