A dual-receptor T-cell platform with Ab-TCR and costimulatory receptor achieves specificity and potency against AML
- PMID: 38048594
- PMCID: PMC10950474
- DOI: 10.1182/blood.2023021054
A dual-receptor T-cell platform with Ab-TCR and costimulatory receptor achieves specificity and potency against AML
Abstract
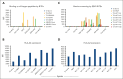
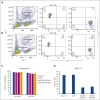
Chimeric antigen receptor T-cell (CAR T) therapy has produced remarkable clinical responses in B-cell neoplasms. However, many challenges limit this class of agents for the treatment of other cancer types, in particular the lack of tumor-selective antigens for solid tumors and other hematological malignancies, such as acute myeloid leukemia (AML), which may be addressed without significant risk of severe toxicities while providing sufficient abundance for efficient tumor suppression. One approach to overcome this hurdle is dual targeting by an antibody-T-cell receptor (AbTCR) and a chimeric costimulatory signaling receptor (CSR) to 2 different antigens, in which both antigens are found together on the cancer cells but not together on normal cells. To explore this proof of concept in AML, we engineered a new T-cell format targeting Wilms tumor 1 protein (WT1) and CD33; both are highly expressed on most AML cells. Using an AbTCR comprising a newly developed TCR-mimic monoclonal antibody against the WT1 RMFPNAPYL (RMF) epitope/HLA-A2 complex, ESK2, and a secondary CSR comprising a single-chain variable fragment directed to CD33 linked to a truncated CD28 costimulatory fragment, this unique platform confers specific T-cell cytotoxicity to the AML cells while sparing healthy hematopoietic cells, including CD33+ myelomonocytic normal cells. These data suggest that this new platform, named AbTCR-CSR, through the combination of a AbTCR CAR and CSR could be an effective strategy to reduce toxicity and improve specificity and clinical outcomes in adoptive T-cell therapy in AML.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: D.A.S. serves on a board of, or has equity in, or income from, Lantheus, Sellas, Iovance, Pfizer, Actinium Pharmaceuticals, OncoPep, Repertoire, Sapience, Atengen, and Eureka Therapeutics. A.K. is a consultant for Novartis, Rgenta, and Blueprint Medicines. T.D. is a consultant for Eureka Therapeutics. A.D. has patents covering CD33 binders for CAR T cells and bispecifics and is on a board, or has equity, or income from NomoCan Pharmaceuticals and PromiCell Therapeutics Inc. The remaining authors declare no competing financial interests.
The current affiliation of A.Y.C. is Regeneron, Tarrytown, NY.
Figures






Comment in
-
Two to tango: engineered T cells against AML.Blood. 2024 Feb 8;143(6):476-478. doi: 10.1182/blood.2023023004. Blood. 2024. PMID: 38329777 No abstract available.
References
-
- Cummins KD, Gill S. Will CAR T cell therapy have a role in AML? Promises and pitfalls. Semin Hematol. 2019;56(2):155–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

