Isochronic development of cortical synapses in primates and mice
- PMID: 38049416
- PMCID: PMC10695974
- DOI: 10.1038/s41467-023-43088-3
Isochronic development of cortical synapses in primates and mice
Abstract
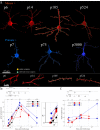
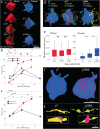
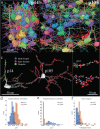
The neotenous, or delayed, development of primate neurons, particularly human ones, is thought to underlie primate-specific abilities like cognition. We tested whether synaptic development follows suit-would synapses, in absolute time, develop slower in longer-lived, highly cognitive species like non-human primates than in shorter-lived species with less human-like cognitive abilities, e.g., the mouse? Instead, we find that excitatory and inhibitory synapses in the male Mus musculus (mouse) and Rhesus macaque (primate) cortex form at similar rates, at similar times after birth. Primate excitatory and inhibitory synapses and mouse excitatory synapses also prune in such an isochronic fashion. Mouse inhibitory synapses are the lone exception, which are not pruned and instead continuously added throughout life. The monotony of synaptic development clocks across species with disparate lifespans, experiences, and cognitive abilities argues that such programs are likely orchestrated by genetic events rather than experience.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Gould, S. J. Ontogeny and Phylogeny (Harvard University Press, 1977).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

