Meningeal interleukin-17-producing T cells mediate cognitive impairment in a mouse model of salt-sensitive hypertension
- PMID: 38049579
- PMCID: PMC10999222
- DOI: 10.1038/s41593-023-01497-z
Meningeal interleukin-17-producing T cells mediate cognitive impairment in a mouse model of salt-sensitive hypertension
Abstract
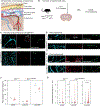
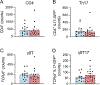
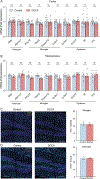
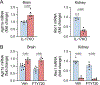
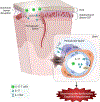
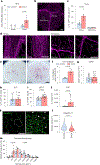
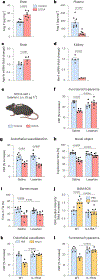
Hypertension (HTN), a disease afflicting over one billion individuals worldwide, is a leading cause of cognitive impairment, the mechanisms of which remain poorly understood. In the present study, in a mouse model of HTN, we find that the neurovascular and cognitive dysfunction depends on interleukin (IL)-17, a cytokine elevated in individuals with HTN. However, neither circulating IL-17 nor brain angiotensin signaling can account for the dysfunction. Rather, IL-17 produced by T cells in the dura mater is the mediator released in the cerebrospinal fluid and activating IL-17 receptors on border-associated macrophages (BAMs). Accordingly, depleting BAMs, deleting IL-17 receptor A in brain macrophages or suppressing meningeal T cells rescues cognitive function without attenuating blood pressure elevation, circulating IL-17 or brain angiotensin signaling. Our data unveil a critical role of meningeal T cells and macrophage IL-17 signaling in the neurovascular and cognitive dysfunction in a mouse model of HTN.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
C.I. is on the scientific advisory board of Broadview Ventures. All other authors declare no competing interests.
Figures

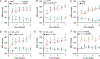









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

