Prostate lineage-specific metabolism governs luminal differentiation and response to antiandrogen treatment
- PMID: 38049604
- PMCID: PMC10709144
- DOI: 10.1038/s41556-023-01274-x
Prostate lineage-specific metabolism governs luminal differentiation and response to antiandrogen treatment
Abstract
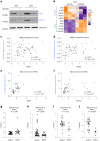
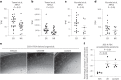
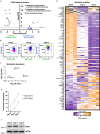
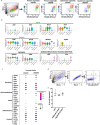
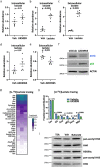
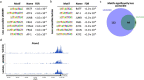
Lineage transitions are a central feature of prostate development, tumourigenesis and treatment resistance. While epigenetic changes are well known to drive prostate lineage transitions, it remains unclear how upstream metabolic signalling contributes to the regulation of prostate epithelial identity. To fill this gap, we developed an approach to perform metabolomics on primary prostate epithelial cells. Using this approach, we discovered that the basal and luminal cells of the prostate exhibit distinct metabolomes and nutrient utilization patterns. Furthermore, basal-to-luminal differentiation is accompanied by increased pyruvate oxidation. We establish the mitochondrial pyruvate carrier and subsequent lactate accumulation as regulators of prostate luminal identity. Inhibition of the mitochondrial pyruvate carrier or supplementation with exogenous lactate results in large-scale chromatin remodelling, influencing both lineage-specific transcription factors and response to antiandrogen treatment. These results establish reciprocal regulation of metabolism and prostate epithelial lineage identity.
© 2023. The Author(s).
Conflict of interest statement
P.C.B. sits on the Scientific Advisory Boards of Sage Bionetworks, BioSymetrics Inc. and Intersect Diagnostics Inc. E.C. obtains funding from Genentech, Sanofi, AbbVie, Astra Zeneca, Foghorn Pharmaceuticals, Kronos Bio, MacroGenics, Janssen Research, Bayer Pharmaceuticals, Forma Pharmaceuticals, Gilead and Zenith Epigenetics, and is a consultant of DotQuant. P.S.N. has received consulting fees from Janssen, Merck, Bristol Myers Squib and Venable Fitzpatrick, and received research funding from Janssen for work unrelated to the present study. All other authors declare no competing interests.
Figures










References
-
- Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. - PubMed
-
- Ousset, M. et al. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat. Cell Biol. 10.1038/ncb2600 (2012). - PubMed
-
- Wang, J. et al. Symmetrical and asymmetrical division analysis provides evidence for a hierarchy of prostate epithelial cell lineages. Nat. Commun.10.1038/ncomms5758 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA237191/CA/NCI NIH HHS/United States
- R21 CA277368/CA/NCI NIH HHS/United States
- TL1 DK132768/DK/NIDDK NIH HHS/United States
- P50 CA097186/CA/NCI NIH HHS/United States
- U2C CA271894/CA/NCI NIH HHS/United States
- R01 CA234715/CA/NCI NIH HHS/United States
- U2C DK129496/DK/NIDDK NIH HHS/United States
- U01 CA224044/CA/NCI NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- U01 CA214194/CA/NCI NIH HHS/United States
- P50 CA092131/CA/NCI NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- P30 DK041301/DK/NIDDK NIH HHS/United States
- T32 GM007185/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

