Thalamic deep brain stimulation in traumatic brain injury: a phase 1, randomized feasibility study
- PMID: 38049620
- PMCID: PMC11087147
- DOI: 10.1038/s41591-023-02638-4
Thalamic deep brain stimulation in traumatic brain injury: a phase 1, randomized feasibility study
Abstract
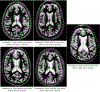
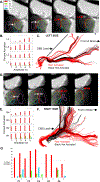
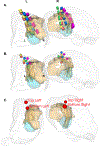
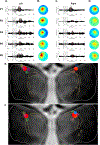
Converging evidence indicates that impairments in executive function and information-processing speed limit quality of life and social reentry after moderate-to-severe traumatic brain injury (msTBI). These deficits reflect dysfunction of frontostriatal networks for which the central lateral (CL) nucleus of the thalamus is a critical node. The primary objective of this feasibility study was to test the safety and efficacy of deep brain stimulation within the CL and the associated medial dorsal tegmental (CL/DTTm) tract.Six participants with msTBI, who were between 3 and 18 years post-injury, underwent surgery with electrode placement guided by imaging and subject-specific biophysical modeling to predict activation of the CL/DTTm tract. The primary efficacy measure was improvement in executive control indexed by processing speed on part B of the trail-making test.All six participants were safely implanted. Five participants completed the study and one was withdrawn for protocol non-compliance. Processing speed on part B of the trail-making test improved 15% to 52% from baseline, exceeding the 10% benchmark for improvement in all five cases.CL/DTTm deep brain stimulation can be safely applied and may improve executive control in patients with msTBI who are in the chronic phase of recovery.ClinicalTrials.gov identifier: NCT02881151 .
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing Interest Statement
The following authors are listed inventors on a patent application WO2023/043786 (jointly filed by Weill Cornell Medicine, University of Utah, and Stanford University) describing detailed methods of integrating magnetic resonance imaging, biophysical modeling and electrophysiological methods for localization and placement of deep brain stimulation electrodes in the CL/DTTm of the human thalamus as described in the manuscript: Nicholas Schiff, Jonathan Baker, Christopher Butson, Andrew Janson, Kyle O’Sullivan, Jaimie Henderson, Eun Young Choi, Brian Rutt, Jason Su, Matthew Radovan. Drs. Schiff, Butson, and Baker are listed inventors on a related patent application WO2021/195062 (jointly filed by Weill Cornell Medicine, University of Utah). Drs. Schiff and Baker are listed as inventors on US Patent 9,9592,383 assigned to Weill Cornell Medicine describing different apparatus but related methods. The remaining authors declare no competing interests.
Figures




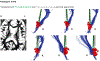



References
-
- Dikmen SS, Machamer JE, Powell JM & Temkin NR Outcome 3 to 5 years after moderate to severe traumatic brain injury. Arch Phys Med Rehabil 84, 1449–1457 (2003). - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

