Neural cell engraftment therapy for sporadic Creutzfeldt-Jakob disease restores neuroelectrophysiological parameters in a cerebral organoid model
- PMID: 38049877
- PMCID: PMC10696693
- DOI: 10.1186/s13287-023-03591-2
Neural cell engraftment therapy for sporadic Creutzfeldt-Jakob disease restores neuroelectrophysiological parameters in a cerebral organoid model
Abstract
Background: Sporadic Creutzfeldt-Jakob disease (sCJD), the most common human prion disease, is a fatal neurodegenerative disease with currently no treatment options. Stem cell therapy for neurodegenerative diseases is emerging as a possible treatment option. However, while there are a few clinical trials for other neurodegenerative disorders such as Parkinson's disease, prion disease cell therapy research has so far been confined to animal models.
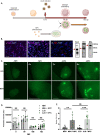
Methods: Here, we use a novel approach to study cell therapies in sCJD using a human cerebral organoid model. Cerebral organoids can be infected with sCJD prions allowing us to assess how neural precursor cell (NPC) therapy impacts the progression of sCJD. After 90 days of sCJD or mock infection, organoids were either seeded with NPCs or left unseeded and monitored for cellular composition changes, prion infection parameters and neuroelectrophysiological function at 180 days post-infection.
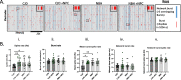
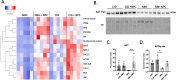
Results: Our results showed NPCs integrated into organoids leading to an increase in neuronal markers and changes in cell signaling irrespective of sCJD infection. Although a small, but significant, decrease in protease-resistant PrP deposition was observed in the CJD-infected organoids that received the NPCs, other disease-associated parameters showed minimal changes. However, the NPCs had a beneficial impact on organoid function following infection. sCJD infection caused reduction in neuronal spike rate and mean burst spike rate, indicative of reduced action potentials. NPC seeding restored these electrophysiological parameters to the uninfected control level.
Conclusions: Together with the previous animal studies, our results support that cell therapy may have some functional benefit for the treatment of human prion diseases.
Keywords: Cell therapy; Cerebral organoid; Electrophysiology; Neural progenitor; Prion; Sporadic CJD.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

