Clonal haematopoiesis across the age spectrum of vasculitis patients with Takayasu's arteritis, ANCA-associated vasculitis and giant cell arteritis
- PMID: 38049983
- PMCID: PMC10939924
- DOI: 10.1136/ard-2023-224933
Clonal haematopoiesis across the age spectrum of vasculitis patients with Takayasu's arteritis, ANCA-associated vasculitis and giant cell arteritis
Abstract
Objectives: Ageing and inflammation are associated with clonal haematopoiesis (CH), the emergence of somatic mutations in haematopoietic cells. This study details CH in patients with systemic vasculitis in association with clinical, haematological and immunological parameters.
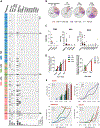
Methods: Patients with three forms of vasculitis were screened for CH in peripheral blood by error-corrected sequencing. Relative contributions of age and vasculitis on CH prevalence were calculated using multivariable logistic regression. Clonal hierarchies were assessed by proteogenomic single-cell DNA sequencing, and functional experiments were performed in association with CH status.
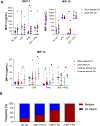
Results: Patients with Takayasu's arteritis (TAK; n=70; mean age=33.2 years), antineutrophil cytoplasmic antibody-associated vasculitis (AAV; n=47; mean age=55.3 years) and giant cell arteritis (GCA; n=59; mean age=71.2 years) were studied. CH, most commonly in DNMT3A and TET2, was detected in 34% (60/176) of patients versus 18% (28/151) of age-matched controls (p<0.01). Prevalence of CH was independently associated with age (standardised B=0.96, p<0.01) and vasculitis (standardised B=0.46, p<0.01), occurring in 61%, 32% and 13% of patients with GCA, AAV and TAK, respectively. Both branched and linear clonal trajectories showed myeloid-lineage bias, and CH was associated with markers of cellular activation. In GCA, mutations were detected in temporal artery biopsies, and clinical relapse correlated with CH in a dose-dependent relationship with clone size.
Conclusions: Age was more strongly associated with CH prevalence than inflammation in systemic vasculitis. Clonal profile was dominated by DNMT3A mutations which were associated with relapse in GCA. CH is not likely a primary causal factor in systemic vasculitis but may contribute to inflammation.
Keywords: Giant Cell Arteritis; Polymorphism, Genetic; Vasculitis.
© Author(s) (or their employer(s)) 2024. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

