Cross-talk between gastric cancer and hepatic stellate cells promotes invadopodia formation during liver metastasis
- PMID: 38050654
- PMCID: PMC10859620
- DOI: 10.1111/cas.16023
Cross-talk between gastric cancer and hepatic stellate cells promotes invadopodia formation during liver metastasis
Abstract
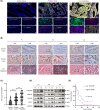
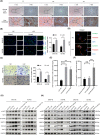
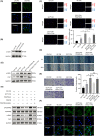
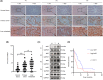
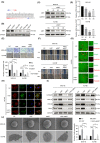
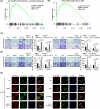
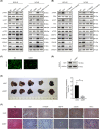
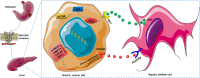
In gastric cancer (GC), the liver is a common organ for distant metastasis, and patients with gastric cancer with liver metastasis (GCLM) generally have poor prognosis. The mechanism of GCLM is unclear. Invadopodia are special membrane protrusions formed by tumor cells that can degrade the basement membrane and ECM. Herein, we investigated the role of invadopodia in GCLM. We found that the levels of invadopodia-associated proteins were significantly higher in liver metastasis than in the primary tumors of patients with GCLM. Furthermore, GC cells could activate hepatic stellate cells (HSCs) within the tumor microenvironment of liver metastases through the secretion of platelet-derived growth factor subunit B (PDGFB). Activated HSCs secreted hepatocyte growth factor (HGF), which activated the MET proto-oncogene, MET receptor of GC cells, thereby promoting invadopodia formation through the PI3K/AKT pathway and subsequently enhancing the invasion and metastasis of GC cells. Therefore, cross-talk between GC cells and HSCs by PDGFB/platelet derived growth factor receptor beta (PDGFRβ) and the HGF/MET axis might represent potential therapeutic targets to treat GCLM.
Keywords: HGF/MET; gastric cancer; hepatic stellate cell; invadopodia; liver metastasis.
© 2023 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. - PubMed
-
- Polkowska‐Pruszynska B, Rawicz‐Pruszynski K, Cisel B, et al. Liver metastases from gastric carcinoma: a case report and review of the literature. Curr Probl Cancer. 2017;41(3):222‐230. - PubMed
-
- Granieri S, Altomare M, Bruno F, et al. Surgical treatment of gastric cancer liver metastases: systematic review and meta‐analysis of long‐term outcomes and prognostic factors. Crit Rev Oncol Hematol. 2021;163:103313. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

