C-N Bond Formation at Discrete CuIII-Aryl Complexes
- PMID: 38050828
- PMCID: PMC11019775
- DOI: 10.1021/jacs.3c09260
C-N Bond Formation at Discrete CuIII-Aryl Complexes
Abstract
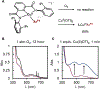
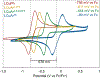
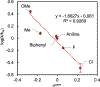
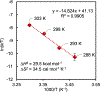
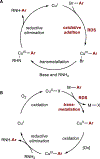
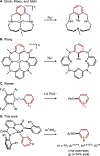
Copper(III) aryl species are widely proposed as intermediates in Cu-catalyzed C-C and C-heteroatom bond formation reactions. Despite their wide utility, mechanistic aspects of C-heteroatom formation at CuIII centers as well as factors that lead to byproducts, e.g., Ar-H, Ar-Ar, remain elusive due to the rarity of discrete CuIII-Ar complexes. Herein, we report the synthesis and reactivity of a series of CuII and CuIII aryl complexes that closely mimic the intermediates in Cu-catalyzed C-N coupling reactions. Copper(II) aryl complexes [TBA][LCuII-ArR] were synthesized via the treatment of CuII with a range of aryl donors, such as ZnAr2R, TMS-ArR, and ArR-Bpin. Oxidation of [TBA][LCuII-ArR] produces formal copper(III) aryl complexes LCuIII-ArR. Treatment of copper(III) aryl complexes with neutral nitrogen nucleophiles produces the C-N coupling product in up to 64% yield, along with commonly observed byproducts, such as Ar-H and Ar-Ar. Hammett analysis of the C-N bond formation performed with various N-nucleophiles shows a ρ value of -1.66, consistent with the electrophilic character of LCuIII-ArR. We propose mechanisms for common side reactions in Cu-catalyzed coupling reactions that lead to the formation of Ar-Ar and Ar-H.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

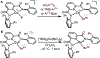
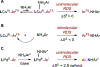

References
-
- Ullmann F; Bielecki J Ueber Synthesen in Der Biphenylreihe. Ber. Dtsch. Chem. Ges 1901, 34, 2174–2185.
-
- Chan DMT; Monaco KL; Wang RP; Winters MP New N- and O-Arylations with Phenylboronic Acids and Cupric Acetate. Tetrahedron Lett. 1998, 39, 2933–2936.
-
- Lam PYS; Bonne D; Vincent G; Clark CG; Combs AP N-Arylation of a-Aminoesters with p-Tolylboronic Acid Promoted by Copper(II) Acetate. Tetrahedron Lett. 2003, 44, 1691–1694.
-
- Lam PYS; Clark CG; Saubern S; Adams J; Winters MP; Chan DMT; Combs A New Aryl/Heteroaryl C-N Bond Cross-Coupling Reactions via Arylboronic Acid/Cupric Acetate Arylation. Tetrahedron Lett. 1998, 39, 2941–2944.
-
- Evans DA; Katz JL; West TR Synthesis of Diaryl Ethers through the Copper-Promoted Arylation of Phenols with Arylboronic Acids. An Expedient Synthesis of Thyroxine. Tetrahedron Lett. 1998, 39, 2937–2940.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

