Breaking barriers in antibody discovery: harnessing divergent species for accessing difficult and conserved drug targets
- PMID: 38050985
- PMCID: PMC10793698
- DOI: 10.1080/19420862.2023.2273018
Breaking barriers in antibody discovery: harnessing divergent species for accessing difficult and conserved drug targets
Abstract
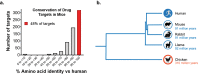
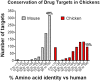
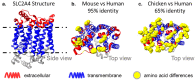
To exploit highly conserved and difficult drug targets, including multipass membrane proteins, monoclonal antibody discovery efforts increasingly rely on the advantages offered by divergent species such as rabbits, camelids, and chickens. Here, we provide an overview of antibody discovery technologies, analyze gaps in therapeutic antibodies that stem from the historic use of mice, and examine opportunities to exploit previously inaccessible targets through discovery now possible in alternate species. We summarize the clinical development of antibodies raised from divergent species, discussing how these animals enable robust immune responses against highly conserved binding sites and yield antibodies capable of penetrating functional pockets via long HCDR3 regions. We also discuss the value of pan-reactive molecules often produced by these hosts, and how these antibodies can be tested in accessible animal models, offering a faster path to clinical development.
Keywords: Camelid antibodies; chicken antibodies; conserved epitopes; divergent species; membrane protein antibodies; pan-reactive antibody; paratope; rabbit antibodies; therapeutic antibody discovery.
Conflict of interest statement
Integral Molecular is a biotech company that uses divergent species for antibody discovery. S.S.R.B., R.C., and B.J.D. are current employees and shareholders of Integral Molecular.
Figures



References
-
- Jain T, Sun T, Durand S, Hall A, Houston NR, Nett JH, Sharkey B, Bobrowicz B, Caffry I, Yu Y, et al. Biophysical properties of the clinical-stage antibody landscape. Proceedings of the National Academy of Sciences of the United States of America. 2017; 114:944–49. doi: 10.1073/pnas.1616408114. PMID: 28096333. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
