Salmonella actively modulates TFEB in murine macrophages in a growth-phase and time-dependent manner
- PMID: 38051049
- PMCID: PMC10783059
- DOI: 10.1128/spectrum.04981-22
Salmonella actively modulates TFEB in murine macrophages in a growth-phase and time-dependent manner
Abstract
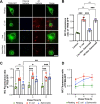
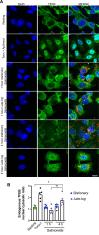
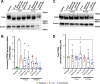
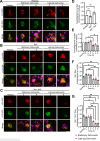
Activation of the host transcription factor TFEB helps mammalian cells adapt to stresses such as starvation and infection by upregulating lysosome, autophagy, and immuno-protective gene expression. Thus, TFEB is generally thought to protect host cells. However, it may also be that pathogenic bacteria like Salmonella orchestrate TFEB in a spatio-temporal manner to harness its functions to grow intracellularly. Indeed, the relationship between Salmonella and TFEB is controversial since some studies showed that Salmonella actively promotes TFEB, while others have observed that Salmonella degrades TFEB and that compounds that promote TFEB restrict bacterial growth. Our work provides a path to resolve these apparent discordant observations since we showed that stationary-grown Salmonella actively delays TFEB after infection, while late-log Salmonella is permissive of TFEB activation. Nevertheless, the exact function of this manipulation remains unclear, but conditions that erase the conditional control of TFEB by Salmonella may be detrimental to the microbe.
Keywords: Salmonella; bacteria; cell adaptation; culture; innate immunity; lysosomes; macrophages; transcription factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
MeSH terms
Substances
Grants and funding
- RGPIN/04679-2015/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (NSERC)
- EQPEQ 458501-2014/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (NSERC)
- 950-232333/Canada Research Chairs (Chaires de recherche du Canada)
- ER13-09-042/MDECEC | Ontario Ministry of Research and Innovation (MRI)
- 32957/Canada Foundation for Innovation (CFI)
LinkOut - more resources
Full Text Sources
Research Materials

