Tapinarof cream 1% once daily for the treatment of adults with mild to severe plaque psoriasis: A novel topical therapy targeting the aryl hydrocarbon receptor
- PMID: 38051146
- PMCID: PMC10996039
- DOI: 10.18553/jmcp.2023.29.12-a.s1
Tapinarof cream 1% once daily for the treatment of adults with mild to severe plaque psoriasis: A novel topical therapy targeting the aryl hydrocarbon receptor
Abstract
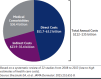
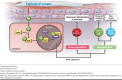
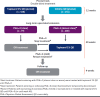
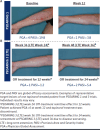
Plaque psoriasis is a chronic, immunemediated skin disease characterized by scaly, erythematous, pruritic plaques. The effects of psoriasis are often debilitating and stigmatizing, significantly impacting patients' physical and psychological well-being and quality of life. Current guideline-recommended psoriasis therapies (topicals, oral systemics, and biologics) have substantial limitations that include overall efficacy, safety, tolerability, sites of application, disease severity, and duration and extent of body surface area treated. Due to these limitations, psoriasis treatment regimens often require combination therapy, especially for moderate to severe disease, leading to increased treatment burden. Psoriasis is also associated with increased indirect costs (eg, reduced work productivity), leading to greater total costs expenditures. Thus, more effective, safe, well-tolerated, and cost-effective therapeutic options are needed. Tapinarof cream 1% once daily is a first-in-class, nonsteroidal, topical aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration in 2022 for the treatment of plaque psoriasis in adults. Tapinarof cream has been evaluated in plaque psoriasis, including 2 pivotal phase 3 trials (NCT03956355 and NCT03983980) and a long-term extension trial (NCT04053387). These trials demonstrated high rates of complete skin clearance with tapinarof cream, durable effects while on treatment (a lack of tachyphylaxis for up to 52 weeks), an approximately 4-month remittive effect off therapy after achieving complete clearance and stopping treatment (ie, duration during which psoriasis does not recur off therapy), and no rebound effects after cessation of therapy. According to the US Food and Drug Administration-approved prescribing information, tapinarof may be used to treat plaque psoriasis of any severity and in any location, has no restrictions on duration of use or extent of total body surface area treated, and has no contraindications, warnings, precautions, or drug-drug interactions. Tapinarof cream is thus an efficacious, well-tolerated, steroid-free topical option that addresses many of the limitations of current recommended therapies. Here we review current knowledge on the physical, psychological, and financial burdens of plaque psoriasis and identify how the clinical profile of tapinarof cream can address key treatment gaps important in the management of plaque psoriasis and patient quality of life. In this article, we aim to assist pharmacists and other managed care practitioners by providing an evidence-based overview of tapinarof cream to support patient-centric decision-making.
Conflict of interest statement
Dr Armstrong is a research investigator and/or scientific advisor for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant Sciences, Inc., Dermira, EPI, Incyte, Janssen, LEO Pharma, Eli Lilly, Modmed, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sun Pharma, Sanofi, and UCB Biopharma. Dr McConaha reports no disclosures. This work was supported by Dermavant Sciences, Inc. Medical writing and editorial assistance were funded by Dermavant Sciences, Inc.
Figures


Similar articles
-
Tapinarof Cream 1% Once Daily for the Treatment of Plaque Psoriasis: Case Photography of Clinical Outcomes from Three Phase 3 Trials.Dermatol Ther (Heidelb). 2023 Oct;13(10):2443-2460. doi: 10.1007/s13555-023-01008-9. Epub 2023 Sep 11. Dermatol Ther (Heidelb). 2023. PMID: 37697121 Free PMC article.
-
Tapinarof, a Novel, First-in-Class, Topical Therapeutic Aryl Hydrocarbon Receptor Agonist for the Management of Psoriasis.J Drugs Dermatol. 2023 Aug 1;22(8):779-784. doi: 10.36849/jdd.7317. J Drugs Dermatol. 2023. PMID: 37556512
-
Phase 3 Trials of Tapinarof Cream for Plaque Psoriasis.N Engl J Med. 2021 Dec 9;385(24):2219-2229. doi: 10.1056/NEJMoa2103629. N Engl J Med. 2021. PMID: 34879448 Clinical Trial.
-
Characteristics and management of follicular events and contact dermatitis in patients using tapinarof cream for the treatment of atopic dermatitis or plaque psoriasis.J Dermatolog Treat. 2025 Dec;36(1):2517388. doi: 10.1080/09546634.2025.2517388. Epub 2025 Jul 2. J Dermatolog Treat. 2025. PMID: 40600584 Review.
-
Tapinarof cream for the topical treatment of plaque psoriasis in adults.Expert Rev Clin Immunol. 2024 Apr;20(4):327-337. doi: 10.1080/1744666X.2023.2296607. Epub 2023 Dec 20. Expert Rev Clin Immunol. 2024. PMID: 38117596 Review.
Cited by
-
Tapinarof Nanogels as a Promising Therapeutic Approach.Pharmaceutics. 2025 Jun 1;17(6):731. doi: 10.3390/pharmaceutics17060731. Pharmaceutics. 2025. PMID: 40574044 Free PMC article. Review.
References
-
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA. 2020;323(19):1945-60. doi:10.1001/jama.2020.4006 - PubMed
-
- Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397(10281):1301-15. doi:10.1016/S0140-6736(20)32549-6 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

