Engagement of CD99 Activates Distinct Programs in Ewing Sarcoma and Macrophages
- PMID: 38051221
- PMCID: PMC10835215
- DOI: 10.1158/2326-6066.CIR-23-0440
Engagement of CD99 Activates Distinct Programs in Ewing Sarcoma and Macrophages
Abstract
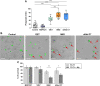
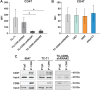
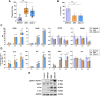
Ewing sarcoma (EWS) is the second most common pediatric bone tumor. The EWS tumor microenvironment is largely recognized as immune-cold, with macrophages being the most abundant immune cells and their presence associated with worse patient prognosis. Expression of CD99 is a hallmark of EWS cells, and its targeting induces inhibition of EWS tumor growth through a poorly understood mechanism. In this study, we analyzed CD99 expression and functions on macrophages and investigated whether the concomitant targeting of CD99 on both tumor and macrophages could explain the inhibitory effect of this approach against EWS. Targeting CD99 on EWS cells downregulated expression of the "don't eat-me" CD47 molecule but increased levels of the "eat-me" phosphatidyl serine and calreticulin molecules on the outer leaflet of the tumor cell membrane, triggering phagocytosis and digestion of EWS cells by macrophages. In addition, CD99 ligation induced reprogramming of undifferentiated M0 macrophages and M2-like macrophages toward the inflammatory M1-like phenotype. These events resulted in the inhibition of EWS tumor growth. Thus, this study reveals what we believe to be a previously unrecognized function of CD99, which engenders a virtuous circle that delivers intrinsic cell death signals to EWS cells, favors tumor cell phagocytosis by macrophages, and promotes the expression of various molecules and cytokines, which are pro-inflammatory and usually associated with tumor regression. This raises the possibility that CD99 may be involved in boosting the antitumor activity of macrophages.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures



References
-
- Grunewald TGP, Cidre-Aranaz F, Surdez D, Tomazou EM, de Alava E, Kovar H, et al. . Ewing sarcoma. Nat Rev Dis Primers 2018;4:5. - PubMed
-
- Balestra T, Manara MC, Laginestra MA, Pasello M, De Feo A, Bassi C, et al. . Targeting CD99 compromises the oncogenic effects of the chimera EWS-FLI1 by inducing reexpression of zyxin and inhibition of GLI1 activity. Mol Cancer Ther 2022;21:58–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

