The crystal structure of the human smacovirus 1 Rep domain
- PMID: 38051309
- PMCID: PMC10833120
- DOI: 10.1107/S2053230X23009536
The crystal structure of the human smacovirus 1 Rep domain
Abstract
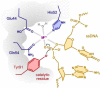
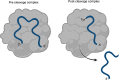
Replication initiator proteins (Reps) from the HUH endonuclease family process specific single-stranded DNA sequences to initiate rolling-circle replication in viruses. Here, the first crystal structure of the apo state of a Rep domain from the smacovirus family is reported. The structure of the human smacovirus 1 Rep domain was obtained at 1.33 Å resolution and represents an expansion of the HUH endonuclease superfamily, allowing greater diversity in bioconjugation-tag applications.
Keywords: HUH endonucleases; HUH-tags; Rep domains; bioconjugation; crystal structure; smacoviruses; ssDNA.
open access.
Figures
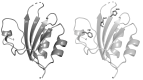


References
-
- Boer, R., Russi, S., Guasch, A., Lucas, M., Blanco, A. G., Pérez-Luque, R., Coll, M. & de la Cruz, F. (2006). J. Mol. Biol. 358, 857–869. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

