Liver Metastases and Immune Checkpoint Inhibitor Efficacy in Patients With Refractory Metastatic Colorectal Cancer: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 38051531
- PMCID: PMC10698621
- DOI: 10.1001/jamanetworkopen.2023.46094
Liver Metastases and Immune Checkpoint Inhibitor Efficacy in Patients With Refractory Metastatic Colorectal Cancer: A Secondary Analysis of a Randomized Clinical Trial
Abstract
Importance: Immune checkpoint inhibitors (ICIs) have limited activity in microsatellite-stable (MSS) or mismatch repair-proficient (pMMR) colorectal cancer. Recent findings suggest the efficacy of ICIs may be modulated by the presence of liver metastases (LM).
Objective: To investigate the association between the presence of LM and ICI activity in advanced MSS colorectal cancer.
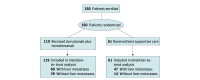
Design, setting, and participants: In this secondary analysis of the Canadian Cancer Trials Group CO26 (CCTG CO.26) randomized clinical trial, patients with treatment-refractory colorectal cancer were randomized in a 2:1 fashion to durvalumab plus tremelimumab or best supportive care alone between August 10, 2016, and June 15, 2017. The primary end point was overall survival (OS) with 80% power and 2-sided α = .10. The median follow-up was 15.2 (0.2-22.0) months. In this post hoc analysis performed from February 11 to 14, 2022, subgroups were defined based on the presence or absence of LM and study treatments.
Intervention: Durvalumab plus tremelimumab or best supportive care.
Main outcomes and measures: Hazard ratios (HRs) and 90% CIs were calculated based on a stratified Cox proportional hazards regression model. Plasma tumor mutation burden at study entry was determined using a circulating tumor DNA assay. The primary end point of the study was OS, defined as the time from randomization to death due to any cause; secondary end points included progression-free survival (PFS) and disease control rate (DCR).
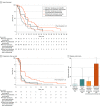
Results: Of 180 patients enrolled (median age, 65 [IQR, 36-87] years; 121 [67.2%] men; 19 [10.6%] Asian, 151 [83.9%] White, and 10 [5.6%] other race or ethnicity), LM were present in 127 (70.6%). For patients with LM, there was a higher proportion of male patients (94 of 127 [74.0%] vs 27 of 53 [50.9%]; P = .005), and the time from initial cancer diagnosis to study entry was shorter (median, 40 [range, 8-153] vs 56 [range, 14-181] months; P = .001). Plasma tumor mutation burden was significantly higher in patients with LM. Patients without LM had significantly improved PFS with durvalumab plus tremelimumab (HR, 0.54 [90% CI, 0.35-0.96]; P = .08; P = .02 for interaction). Disease control rate was 49% (90% CI, 36%-62%) in patients without LM treated with durvalumab plus tremelimumab, compared with 14% (90% CI, 6%-38%) in those with LM (odds ratio, 5.70 [90% CI, 1.46-22.25]; P = .03). On multivariable analysis, patients without LM had significantly improved OS and PFS compared with patients with LM.
Conclusions and relevance: In this secondary analysis of the CCTG CO.26 study, the presence of LM was associated with worse outcomes for patients with advanced colorectal cancer. Patients without LM had improved PFS and higher DCR with durvalumab plus tremelimumab. Liver metastases may be associated with poor outcomes of ICI treatment in advanced colorectal cancer and should be considered in the design and interpretation of future clinical studies evaluating this therapy.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

