Bridging therapy with axicabtagene ciloleucel for large B-cell lymphoma: results from the US Lymphoma CAR-T Consortium
- PMID: 38051550
- PMCID: PMC10920102
- DOI: 10.1182/bloodadvances.2023011489
Bridging therapy with axicabtagene ciloleucel for large B-cell lymphoma: results from the US Lymphoma CAR-T Consortium
Abstract
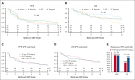
During the manufacturing period of autologous chimeric antigen receptor (CAR) T-cell therapy, patients may experience a decline in their condition due to cancer progression. In this study, we investigated the impact of bridging therapy (BT) on the outcome of patients with relapsed/refractory large B-cell lymphoma who received antilymphoma treatment between leukapheresis and axicabtagene ciloleucel (axi-cel) infusion. We conducted our analysis using data from the multicenter US Lymphoma CAR-T Consortium, with a median follow-up of 33 months (range, 4.3-42.1). Out of the 298 patients who underwent leukapheresis, 275 patients received axi-cel. A total 52% of patients (n = 143) who received BT had a higher baseline risk profile than patients who did not receive BT, and these patients, as a group, had inferior outcomes compared with those who did not receive BT. However, after propensity score matching between the 2 groups, there were no statistically significant differences in overall response rate (77% vs 87%; P = .13), complete response rate (58% vs 70%; P = .1), progression-free survival (hazard ratio [HR], 1.25; P = .23), and overall survival (HR, 1.39; P=.09) between the BT group and the no-BT group, respectively. Analyzing the effects of BT in the whole cohort that underwent leukapheresis regardless of receiving axi-cel (intention-to-treat analysis) showed similar results. Radiation BT resulted in outcomes similar to those observed with nonradiation BT. Our findings suggest that BT may be safe without a significant impact on long-term survival for patients who require disease stabilization during the manufacturing period. Moreover, our results suggest that there is no clear advantage to using radiation-based BT over nonradiation-based BT.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.D.J. reports consultancy/advisory for Kite/Gilead and Myeloid Therapeutics and received research funding from Kite/Gilead, Incyte, and Loxo@Lilly. L.J.N. has received honorarium for participation in advisory boards/consulting from AbbVie, ADC Therapeutics, Atara Biotherapeutics, Bristol Myers Squibb (BMS)/Celgene, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech/Roche, Genmab, Gilead/Kite, Janssen, Incyte, Merck, Novartis, and Takeda and received research support from BMS/Celgene, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech/Roche, Genmab, Gilead/Kite, IGM Biosciences, Janssen, Novartis, and Takeda. Y.L. reports consulting or advisory role at Kite/Gilead, Novartis, bluebird bio, Celgene, Juno Therapeutics, BMS, Gamida Cell, Legend Biotech, Sorrento Therapeutics, Vineti, Janssen Oncology, and Pfizer (all to institution); and received research funding from Janssen Oncology, Janssen Oncology, Merck, Takeda, Boston Scientific, Kite/Gilead, BMS, and bluebird bio (all to institution). S.D. reports consulting or advisory role at Kite/Gilead. M.L. reports consultancy/advisory for AbbVie, Acrotech, ADC Therapeutics, AstraZeneca, Astellas, BMS, Caribou, CRISPR, Daiichi Sankyo, EUSA, Fate Therapeutics, Genentech, Genmab, Instil Bio, Ipsen, Janssen, Kite, Loxo, Miltenyi, MorphoSys, Novartis, Nurix, Pharmacyclics, Regeneron, Sanofi, Seagen, and Takeda, TG Therapeutics and research Funding for BMS and Curis. P.R. received research support from Seagen and Seattle Genetics and consulting for Kite Pharma-Gilead and Caribou Biosciences. O.O. reports consulting or advisory role at Kite/Gilead, Legend Biotech, Curio Science, Novartis, ADC Therapeutics, Syncopation Life Sciences, Nektar, Gilead Sciences, and Epizyme and received research Funding from Kite, a Gilead company (to the institution). J.M. reports consulting/advisory for AlloVir, Novartis, Kite, and BMS. A.D. reports consulting or advisory role at Novartis, Kite/Gilead, Agios, Juno/Celgene, Janssen, and Adicet Bio. A.R.S. received research funding from Kite Pharma–Gilead and BMS–Juno Therapeutics. A.G. received grant/research support as a principal investigator (research to institution) for Acerta, AstraZeneca, BMS, Celgene, Genentech, Infinity Pharmaceuticals, Janssen, Janssen Global Services, Karyopharm, Kite Pharma, Pharmacyclics, Seattle Genetics, and Verastem; is a stock/shareholder of COTA, Alloplex, Resilience, and Genomic Testing Cooperative; is a consultant at Pharmacyclics LLC, an AbbVie Company, and Janseen Global Services LLC, AbbVie, Clinical Advances in Hematology & Oncology, Novartis, Michael J. Hennessey Associates, Novartis, and BMS; is on the Advisory board of Kite, Janseen Biotech, and Pharmacyclics LLC; is a speaker at Physicians Education Resource, LLC and third GCC Hematology Expert Forum; is on the board of directors at COTA, Resilience, Genomic Testing Cooperative; is a consulting faculty at Michael J Hennessey Associates, , and Physcians Education Resource, LLC; is on the scientific advisory board at Alloplex, BMS, and Vincerx; is on the steering committee at Astrazenca, Pharmacyclics LLC, an AbbVie Company, and Janssen Biotech. C.A. reports consulting or advisory role at Gilead Sciences, Kite, a Gilead company, Karyopharm Therapeutics, Atara Biotherapeutics, Incyte, TG therapeutics, and Epizyme and received research funding from Novartis, Merck, BMS, and Genmab. J.M. received honoraria from Kyowa Hakko Kirin, Seattle Genetics, Targeted Oncology, Onc view, Curio Science, and Physicians' Education Resource; reports a consulting or advisory role at Kite, a Gilead company, Pfizer, Pharmacyclics, Bayer, Alexion Pharmaceuticals, BMS, Janssen, Seattle Genetics, Gilead Sciences, Kyowa Hakko Kirin, Juno Therapeutics, Genentech, Celgene, BeiGene, Fosun Kite, Innovent Biologics, Debiopharm Group, Karyopharm Therapeutics, Genmab, ADC Therapeutics, Epizyme, Servier, Novartis, MorphoSys, Aurobindo, Lilly, and Secura Bio; is on the speakers' bureau at Kite, a Gilead company, Bayer, Pharmacyclics/Janssen, AstraZeneca, Gilead Sciences, Seattle Genetics, Kyowa Hakko Kirin, Acrotech Biopharma, BeiGene, Verastem, Celgene, and AbbVie/Genentech; and received research funding from Kite, a Gilead company, Celgene, Portola Pharmaceuticals, Incyte, Genentech/AbbVie, Pharmacyclics/Janssen, Seattle Genetics, and Millennium. J.C.C. reports consultancy/advisory/honoraria for Kite/Gilead, Novartis, Karyopharm, MorphoSys, BeiGene, AbbVie, ADC Therapeutics, BMS, Epizyme, Genentech, and Bayer and research funding from AstraZeneca, Merck, and Adaptive. N.N.B. received research funding from Kite Pharma-Gilead and Affimed and is an advisory board member for Daiichi Sankyo, Verastem, and Purdue Pharma. J.M.V. received honoraria from Acerta Pharma/AstraZeneca, MorphoSys, Johnson and Johnson, MEI Pharma, Lilly, AbbVie, and Merck; received research funding from Celgene, Incyte, Acerta Pharma, Kite, a Gilead company, Seattle Genetics, Novartis, BMS, AstraZeneca (all to institution), Loxo, and Epizyme. D.B.M. received honoraria from Janssen and Fosun Kite Biotechnology; reports consulting or advisory Role at Adaptive Biotechnologies, Juno/Celgene, Pharmacyclics, and Janssen; received research funding from Pharmacyclics, Novartis, Roche/Genentech, Kite, a Gilead company, Adaptive Biotechnologies, Alimera Sciences, Precision Biosciences, and Adicet Bio; reports patent held with Pharmacyclics supporting ibrutinib for chronic graft-versus-host disease (no royalty claim). S.S.N. holds stock and other ownership interests in Longbow Immunotherapy Inc; received honoraria from Bio Ascend, Medscape, Aptitude Health, and MJH Life Sciences; reports consulting or advisory role at Merck Sharp & Dohme, Kite, a Gilead company, Novartis, Incyte, Gilead Sciences, Alimera Sciences, BMS, Adicet Bio, Calibr, Athenex, Sellas Life Sciences, bluebird bio, and Sana Biotechnology; received research funding from BMS, Kite, a Gilead company, Cellectis, Poseida Therapeutics, Unum Therapeutics, Gilead Sciences, Alimera Sciences, Precision BioSciences, and Adicet Bio; and reports patents related to cellular therapy and royalty income from Takeda Pharmaceuticals. F.L.L. reports consulting or advisory Role at Novartis, Celgene, Calibr, Alimera Sciences, Gerson Lehrman Group, EcoR1 Capital, Amgen, bluebird bio, BMS, Iovance Biotherapeutics, Legend Biotech, Cowen, Kite, a Gilead company, Umoja Biopharma, Takeda, Sana Biotechnology, Daiichi Sankyo/UCB Japan, BMS/Celgene, Janssen, A2 Biotherapeutics, Miltenyi Biotec, Caribou Biosciences, Takeda, and Umoja Biopharma; received research funding from Kite, a Gilead company, Alimera Sciences, Novartis, bluebird bio, BMS/Celgene (all to the institution); reports patents, royalties, and other intellectual property (all to the institution): Double Mutant Survivin Vaccine, US010414810B2; CAR T cells with enhanced metabolic fitness, serial no.: 62/939 727; methods of enhancing CAR T-cell therapies, serial no.: 62/892 292; evolutionary dynamics of non-Hodgkin lymphoma CAR T-Cell therapy, serial no.: 62/879 534; and received travel, accommodation and expenses from Kite, a Gilead company, and A2 Biotherapeutics. A.G. received research support from Kite Pharma-Gilead and Amgen and is an advisory board member/consultant for Kite Pharma-Gilead, Amgen, Celgene, EUSA, Atara, CRISPR Therapeutics, and Wugen. The remaining authors declare no competing financial interests.
Figures



Comment in
-
Bridging therapy before axi-cel for lymphoma.Blood Adv. 2024 Feb 27;8(4):1051-1052. doi: 10.1182/bloodadvances.2023012128. Blood Adv. 2024. PMID: 38411993 Free PMC article. No abstract available.
References
-
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. - PubMed

