Atorvastatin for patients with cirrhosis. A randomized, placebo-controlled trial
- PMID: 38051553
- PMCID: PMC10697620
- DOI: 10.1097/HC9.0000000000000332
Atorvastatin for patients with cirrhosis. A randomized, placebo-controlled trial
Abstract
Background: Patients with cirrhosis and portal hypertension face a high risk of complications. Besides their anti-inflammatory and antifibrotic effects, statins may reduce portal pressure and thus the risk of complications and mortality. We aimed to investigate the effects of atorvastatin on hospital admissions, mortality, inflammation, and lipidomics in cirrhosis with portal hypertension.
Methods: We performed a double-blinded, randomized, placebo-controlled clinical trial among patients with cirrhosis and portal hypertension. Atorvastatin (10-20 mg/d) was administered for 6 months. We measured splanchnic hemodynamics, analyzed inflammatory markers, and performed lipidomics at baseline and after 6 months.
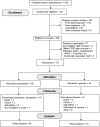
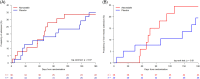
Results: Seventy-eight patients were randomized, with 38 patients allocated to atorvastatin and 40 patients to placebo. Fifty-nine patients completed 6 months of intervention. Comparisons between changes in each group were calculated. Liver-related complications and mortality were similar between the groups. The HVPG and Model for End-stage Liver Disease score did not change between groups (p=0.95 and 0.87, respectively). Atorvastatin decreased 3 of 42 inflammatory markers, CD62-L-selectin, matrix metalloproteinases-2, and TNF-α (p-values: 0.005, 0.011, and 0.023, respectively), while lipidomics was not significantly changed.
Conclusions: In patients with cirrhosis, atorvastatin was safe to use, but did not reduce mortality, the risk of liver-related complications, or the HVPG. Atorvastatin induced minor anti-inflammatory effects and minor effects on lipids during a 6-month treatment period.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Henning Grønbæk consults for AstraZeneca and Novo Nordisk. He received grants from AbbVie and Intercept. The remaining authors have no conflicts to report.
Figures
Comment in
-
Statin the course: Navigating unchartered territory in cirrhosis.Hepatol Commun. 2024 Jun 3;8(6):e0456. doi: 10.1097/HC9.0000000000000456. eCollection 2024 Jun 1. Hepatol Commun. 2024. PMID: 38829204 Free PMC article. No abstract available.
References
-
- Fleming KM, Aithal GP, Card TR, West J. All-cause mortality in people with cirrhosis compared with the general population: A population-based cohort study. Liver Int. 2012;32:79–84. - PubMed
-
- Deleuran T, Vilstrup H, Jepsen P. Decreasing mortality among Danish alcoholic cirrhosis patients: A nationwide cohort study. Am J Gastroenterol. 2016;111:817–22. - PubMed
-
- Costa D, Simbrunner B, Jachs M, Hartl L, Bauer D, Paternostro R, et al. . Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J Hepatol. 2021;74:819–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical