Transcriptomic and functional screening of weapon formation genes implies significance of cell adhesion molecules and female-biased genes in broad-horned flour beetle
- PMID: 38051754
- PMCID: PMC10723671
- DOI: 10.1371/journal.pgen.1011069
Transcriptomic and functional screening of weapon formation genes implies significance of cell adhesion molecules and female-biased genes in broad-horned flour beetle
Abstract
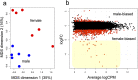
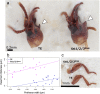
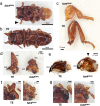
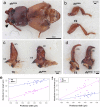
For understanding the evolutionary mechanism of sexually selected exaggerated traits, it is essential to uncover its molecular basis. By using broad-horned flour beetle that has male-specific exaggerated structures (mandibular horn, head horn and gena enlargement), we investigated the transcriptomic and functional characters of sex-biased genes. Comparative transcriptome of male vs. female prepupal heads elucidated 673 sex-biased genes. Counter-intuitively, majority of them were female-biased (584 genes), and GO enrichment analysis showed cell-adhesion molecules were frequently female-biased. This pattern motivated us to hypothesize that female-biased transcripts (i.e. the transcripts diminished in males) may play a role in outgrowth formation. Potentially, female-biased genes may act as suppressors of weapon structure. In order to test the functionality of female-biased genes, we performed RNAi-mediated functional screening for top 20 female-biased genes and 3 genes in the most enriched GO term (cell-cell adhesion, fat1/2/3, fat4 and dachsous). Knockdown of one transcription factor, zinc finger protein 608 (zfp608) resulted in the formation of male-like gena in females, supporting the outgrowth suppression function of this gene. Similarly, knockdown of fat4 induced rudimental, abnormal mandibular horn in female. fat1/2/3RNAi, fat4RNAi and dachsousRNAi males exhibited thick and/or short mandibular horns and legs. These cell adhesion molecules are known to regulate tissue growth direction and known to be involved in the weapon formation in Scarabaeoidea beetles. Functional evidence in phylogenetically distant broad-horned flour beetle suggest that cell adhesion genes are repeatedly deployed in the acquisition of outgrowth. In conclusion, this study clarified the overlooked functions of female-biased genes in weapon development.
Copyright: © 2023 Sugiyama et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Andersson MB. Sexual selection. Princeton University Press; 1994.
-
- Darwin C. The descent of man: and selection in relation to sex. 1st ed. John Murray, London.; 1871.
-
- Zimmer C, Emlen DJ, Perkins AEH. Evolution: making sense of life. Roberts; 2016.
-
- Emlen DJ. The Evolution of Animal Weapons. Annu Rev Ecol Evol Syst. 2008;39: 387–413. doi: 10.1146/annurev.ecolsys.39.110707.173502 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

