Alternative splicing events as peripheral biomarkers for motor learning deficit caused by adverse prenatal environments
- PMID: 38051767
- PMCID: PMC10723155
- DOI: 10.1073/pnas.2304074120
Alternative splicing events as peripheral biomarkers for motor learning deficit caused by adverse prenatal environments
Abstract
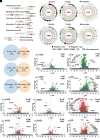
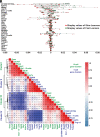
Severity of neurobehavioral deficits in children born from adverse pregnancies, such as maternal alcohol consumption and diabetes, does not always correlate with the adversity's duration and intensity. Therefore, biological signatures for accurate prediction of the severity of neurobehavioral deficits, and robust tools for reliable identification of such biomarkers, have an urgent clinical need. Here, we demonstrate that significant changes in the alternative splicing (AS) pattern of offspring lymphocyte RNA can function as accurate peripheral biomarkers for motor learning deficits in mouse models of prenatal alcohol exposure (PAE) and offspring of mother with diabetes (OMD). An aptly trained deep-learning model identified 29 AS events common to PAE and OMD as superior predictors of motor learning deficits than AS events specific to PAE or OMD. Shapley-value analysis, a game-theory algorithm, deciphered the trained deep-learning model's learnt associations between its input, AS events, and output, motor learning performance. Shapley values of the deep-learning model's input identified the relative contribution of the 29 common AS events to the motor learning deficit. Gene ontology and predictive structure-function analyses, using Alphafold2 algorithm, supported existing evidence on the critical roles of these molecules in early brain development and function. The direction of most AS events was opposite in PAE and OMD, potentially from differential expression of RNA binding proteins in PAE and OMD. Altogether, this study posits that AS of lymphocyte RNA is a rich resource, and deep-learning is an effective tool, for discovery of peripheral biomarkers of neurobehavioral deficits in children of diverse adverse pregnancies.
Keywords: RNA splicing; machine learning; offspring of mother with diabetes; peripheral biomarker; prenatal alcohol exposure.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
-
- Levitt P., Reinoso B., Jones L., The critical impact of early cellular environment on neuronal development. Prev. Med. 27, 180–183 (1998). - PubMed
-
- Shonkoff J. P., Boyce W. T., Levitt P., Martinez F. D., McEwen B., Leveraging the biology of adversity and resilience to transform pediatric practice. Pediatrics 147, e20193845 (2021). - PubMed
-
- Popova S., Lange S., Probst C., Gmel G., Rehm J., Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob. Health 5, e290–e299 (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

