Triggered lattice-oxygen oxidation with active-site generation and self-termination of surface reconstruction during water oxidation
- PMID: 38051768
- PMCID: PMC10723130
- DOI: 10.1073/pnas.2312224120
Triggered lattice-oxygen oxidation with active-site generation and self-termination of surface reconstruction during water oxidation
Abstract
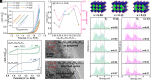
To master the activation law and mechanism of surface lattice oxygen for the oxygen evolution reaction (OER) is critical for the development of efficient water electrolysis. Herein, we propose a strategy for triggering lattice-oxygen oxidation and enabling non-concerted proton-electron transfers during OER conditions by substituting Al in La0.3Sr0.7CoO3-δ. According to our experimental data and density functional theory calculations, the substitution of Al can have a dual effect of promoting surface reconstruction into active Co oxyhydroxides and activating deprotonation on the reconstructed oxyhydroxide, inducing negatively charged oxygen as an active site. This leads to a significant improvement in the OER activity. Additionally, Al dopants facilitate the preoxidation of active cobalt metal, which introduces great structural flexibility due to elevated O 2p levels. As OER progresses, the accumulation of oxygen vacancies and lattice-oxygen oxidation on the catalyst surface leads to the termination of Al3+ leaching, thereby preventing further reconstruction. We have demonstrated a promising approach to achieving tunable electrochemical reconstruction by optimizing the electronic structure and gained a fundamental understanding of the activation mechanism of surface oxygen sites.
Keywords: lattice-oxygen oxidation; oxygen evolution reaction; perovskite oxide; self-termination; surface reconstruction.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
-
- Hwang J., et al. , Perovskites in catalysis and electrocatalysis. Science 358, 751–756 (2017). - PubMed
-
- Hong W. T., et al. , Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 8, 1404–1427 (2015).
-
- Wu T., et al. , Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat. Catal. 2, 763–772 (2019).
-
- Hansen O., Seger B., Vesborg P. C. K., Chorkendorff I., A quick look at how photoelectrodes work. Science 350, 1030–1031 (2015). - PubMed
-
- Castelli I. E., et al. , New cubic perovskites for one- and two-photon water splitting using the computational materials repository. Energy Environ. Sci. 5, 9034–9043 (2012).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

