The TLR2/TLR6 ligand FSL-1 mitigates radiation-induced hematopoietic injury in mice and nonhuman primates
- PMID: 38051771
- PMCID: PMC10723152
- DOI: 10.1073/pnas.2122178120
The TLR2/TLR6 ligand FSL-1 mitigates radiation-induced hematopoietic injury in mice and nonhuman primates
Abstract
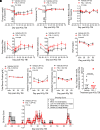
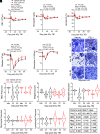
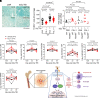
Thrombocytopenia, hemorrhage, anemia, and infection are life-threatening issues following accidental or intentional radiation exposure. Since few therapeutics are available, safe and efficacious small molecules to mitigate radiation-induced injury need to be developed. Our previous study showed the synthetic TLR2/TLR6 ligand fibroblast stimulating lipopeptide (FSL-1) prolonged survival and provided MyD88-dependent mitigation of hematopoietic acute radiation syndrome (H-ARS) in mice. Although mice and humans differ in TLR number, expression, and function, nonhuman primate (NHP) TLRs are like those of humans; therefore, studying both animal models is critical for drug development. The objectives of this study were to determine the efficacy of FSL-1 on hematopoietic recovery in small and large animal models subjected to sublethal total body irradiation and investigate its mechanism of action. In mice, we demonstrate a lack of adverse effects, an easy route of delivery (subcutaneous) and efficacy in promoting hematopoietic progenitor cell proliferation by FSL-1. NHP given radiation, followed a day later with a single subcutaneous administration of FSL-1, displayed no adversity but showed elevated hematopoietic cells. Our analyses revealed that FSL-1 promoted red blood cell development and induced soluble effectors following radiation exposure. Cytologic analysis of bone marrow aspirates revealed a striking enhancement of mononuclear progenitor cells in FSL-1-treated NHP. Combining the efficacy of FSL-1 in promoting hematopoietic cell recovery with the lack of adverse effects induced by a single administration supports the application of FSL-1 as a viable countermeasure against H-ARS.
Keywords: animal models; hematopoiesis; innate immunity; mitigator; radiation.
Conflict of interest statement
Competing interests statement:J.P.Y.T. is a cofounder of and stockholder in IMMvention Therapeutix, which is developing inflammasome inhibitors. US20200282006A1 United States Methods and treatments using toll-like receptor agonists to mitigate hematopoietic myeloid loss, increase gastrointestinal recovery and reduce tumor growth for J.P.Y.T., W.J.B., and H.G.
Figures

References
-
- Farese A. M., MacVittie T. J., Filgrastim for the treatment of hematopoietic acute radiation syndrome. Drugs Today (Barc) 51, 537–548 (2015). - PubMed
-
- McGettrick A. F., O’Neill L. A., Toll-like receptors: Key activators of leucocytes and regulator of haematopoiesis. Br J. Haematol. 139, 185–193 (2007). - PubMed
-
- Vijay-Kumar M., et al. , Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J. Immunol. 180, 8280–8285 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

