Homer1a reduces inflammatory response after retinal ischemia/reperfusion injury
- PMID: 38051906
- PMCID: PMC10883521
- DOI: 10.4103/1673-5374.386490
Homer1a reduces inflammatory response after retinal ischemia/reperfusion injury
Abstract
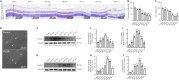
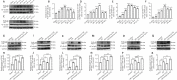
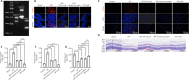
Elevated intraocular pressure (IOP) is one of the causes of retinal ischemia/reperfusion injury, which results in NLRP3 inflammasome activation and leads to visual damage. Homer1a is reported to play a protective role in neuroinflammation in the cerebrum. However, the effects of Homer1a on NLRP3 inflammasomes in retinal ischemia/reperfusion injury caused by elevated IOP remain unknown. In our study, animal models were constructed using C57BL/6J and Homer1flox/–/Homer1a+/–/Nestin-Cre+/– mice with elevated IOP-induced retinal ischemia/reperfusion injury. For in vitro experiments, the oxygen-glucose deprivation/reperfusion injury model was constructed with Müller cells. We found that Homer1a overexpression ameliorated the decreases in retinal thickness and Müller cell viability after ischemia/reperfusion injury. Furthermore, Homer1a knockdown promoted NF-κB P65Ser536 activation via caspase-8, NF-κB P65 nuclear translocation, NLRP3 inflammasome formation, and the production and processing of interleukin-1β and interleukin-18. The opposite results were observed with Homer1a overexpression. Finally, the combined administration of Homer1a protein and JSH-23 significantly inhibited the reduction in retinal thickness in Homer1flox/–/Homer1a+/–/Nestin-Cre+/– mice and apoptosis in Müller cells after ischemia/reperfusion injury. Taken together, these studies demonstrate that Homer1a exerts protective effects on retinal tissue and Müller cells via the caspase-8/NF-κB P65/NLRP3 pathway after I/R injury.
Conflict of interest statement
Figures







Similar articles
-
Homer1a Attenuates Endoplasmic Reticulum Stress-Induced Mitochondrial Stress After Ischemic Reperfusion Injury by Inhibiting the PERK Pathway.Front Cell Neurosci. 2019 Mar 15;13:101. doi: 10.3389/fncel.2019.00101. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30930751 Free PMC article.
-
Retraction notice to "The protective role of sulforaphane and Homer1a in retinal ischemia-reperfusion injury: Unraveling the neuroprotective interplay" [Life Sci. 329 (2023) 121968].Life Sci. 2024 May 15;345:122590. doi: 10.1016/j.lfs.2024.122590. Epub 2024 Mar 28. Life Sci. 2024. PMID: 38553273 No abstract available.
-
Argon reduces microglial activation and inflammatory cytokine expression in retinal ischemia/reperfusion injury.Neural Regen Res. 2021 Jan;16(1):192-198. doi: 10.4103/1673-5374.290098. Neural Regen Res. 2021. PMID: 32788476 Free PMC article.
-
Single-cell RNA sequencing reveals a landscape and targeted treatment of ferroptosis in retinal ischemia/reperfusion injury.J Neuroinflammation. 2022 Oct 26;19(1):261. doi: 10.1186/s12974-022-02621-9. J Neuroinflammation. 2022. PMID: 36289494 Free PMC article.
-
Cellular Stress Response and Immune Signaling in Retinal Ischemia-Reperfusion Injury.Front Immunol. 2016 Oct 24;7:444. doi: 10.3389/fimmu.2016.00444. eCollection 2016. Front Immunol. 2016. PMID: 27822213 Free PMC article. Review.
Cited by
-
Müller cells are activated in response to retinal outer nuclear layer degeneration in rats subjected to simulated weightlessness conditions.Neural Regen Res. 2025 Jul 1;20(7):2116-2128. doi: 10.4103/NRR.NRR-D-23-01035. Epub 2024 Mar 1. Neural Regen Res. 2025. PMID: 39254570 Free PMC article.
-
Key immune regulators in retinal ischemia-reperfusion injury via RNA sequencing.Int J Ophthalmol. 2025 Jul 18;18(7):1237-1251. doi: 10.18240/ijo.2025.07.06. eCollection 2025. Int J Ophthalmol. 2025. PMID: 40688775 Free PMC article.
-
Temporal Trends in Serum Homer1 Levels and Their Prognostic Implications in Aneurysmal Subarachnoid Hemorrhage: A Prospective Cohort Study.Int J Gen Med. 2025 Feb 1;18:567-584. doi: 10.2147/IJGM.S508325. eCollection 2025. Int J Gen Med. 2025. PMID: 39911298 Free PMC article.
-
Dual effects of GABA A R agonist anesthetics in neurodevelopment and vulnerable brains: From neurotoxic to therapeutic effects.Neural Regen Res. 2026 Jan 1;21(1):81-95. doi: 10.4103/NRR.NRR-D-24-00828. Epub 2024 Dec 7. Neural Regen Res. 2026. PMID: 39665822 Free PMC article.
-
Voltage-dependent anion channel 1 oligomerization regulates PANoptosis in retinal ischemia-reperfusion injury.Neural Regen Res. 2026 Apr 1;21(4):1652-1664. doi: 10.4103/NRR.NRR-D-24-00674. Epub 2025 Jan 13. Neural Regen Res. 2026. PMID: 39819824 Free PMC article.
References
-
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. - PubMed
-
- Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. - PubMed
-
- Bay V, Butt AM. Relationship between glial potassium regulation and axon excitability: a role for glial Kir4.1 channels. Glia. 2012;60:651–660. - PubMed
LinkOut - more resources
Full Text Sources

