Phase II study of bemnifosbuvir in high-risk participants in a hospital setting with moderate COVID-19
- PMID: 38051993
- PMCID: PMC10308776
- DOI: 10.2217/fvl-2023-0064
Phase II study of bemnifosbuvir in high-risk participants in a hospital setting with moderate COVID-19
Abstract
Background: Bemnifosbuvir, a novel, oral, nonmutagenic, nonteratogenic nucleotide analogue inhibits SARS-CoV-2 replication in vitro.
Materials & methods: Adults in hospital settings with moderate COVID-19 were randomized 1:1 bemnifosbuvir/placebo. Study amended to two parts after interim analysis; part B enrollment limited owing to evolving standard of care.
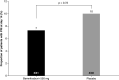
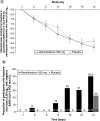
Results: Although the study ended early and did not meet the primary efficacy end point, bemnifosbuvir was well tolerated and did not contribute to all-cause mortality. Compared with placebo, bemnifosbuvir treatment resulted in 0.61 log10 greater viral load mean change on day 2; trend sustained through day 8. Treatment-emergent adverse events were similar in both groups; most were mild/moderate, unrelated to study drug.
Conclusion: Our results suggest a potential role for bemnifosbuvir in blunting COVID-19 progression.
Clinical trial registration: NCT04396106 (ClinicalTrials.gov).
Keywords: AT-527; COVID-19; SARS-CoV-2; bemnifosbuvir; oral.
© 2023 The Authors.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures

References
-
- Salian VS, Wright JA, Vedell PT et al. COVID-19 transmission, current treatment, and future therapeutic strategies. Mol. Pharm. 18(3), 754–771 (2021). - PubMed
-
- Di Fusco M, Shea KM, Lin J et al. Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. J. Med. Econ. 24(1), 308–317 (2021). - PubMed
-
- Iuliano AD, Brunkard JM, Boehmer TK et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods – United States, December 2020–January 2022. Morb. Mortal. Wkly. Rep. 71(4), 146–152 (2022). - PMC - PubMed
-
- World Health Organization. Therapeutics and COVID-19: Living Guideline by World Health Organization. WHO; (2021). https://apps.who.int/iris/handle/10665/345356 - PubMed
-
- FDA press release. FDA Approves First COVID-19 Vaccine. News Release. US FDA; (2021). www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-...
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
