Phase 1b trial of tagraxofusp in combination with azacitidine with or without venetoclax in acute myeloid leukemia
- PMID: 38052038
- PMCID: PMC10837492
- DOI: 10.1182/bloodadvances.2023011721
Phase 1b trial of tagraxofusp in combination with azacitidine with or without venetoclax in acute myeloid leukemia
Abstract
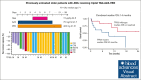
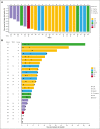
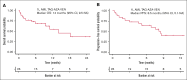
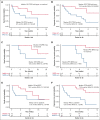
CD123, a subunit of the interleukin-3 receptor, is expressed on ∼80% of acute myeloid leukemias (AMLs). Tagraxofusp (TAG), recombinant interleukin-3 fused to a truncated diphtheria toxin payload, is a first-in-class drug targeting CD123 approved for treatment of blastic plasmacytoid dendritic cell neoplasm. We previously found that AMLs with acquired resistance to TAG were re-sensitized by the DNA hypomethylating agent azacitidine (AZA) and that TAG-exposed cells became more dependent on the antiapoptotic molecule BCL-2. Here, we report a phase 1b study in 56 adults with CD123-positive AML or high-risk myelodysplastic syndrome (MDS), first combining TAG with AZA in AML/MDS, and subsequently TAG, AZA, and the BCL-2 inhibitor venetoclax (VEN) in AML. Adverse events with 3-day TAG dosing were as expected, without indication of increased toxicity of TAG or AZA+/-VEN in combination. The recommended phase 2 dose of TAG was 12 μg/kg/day for 3 days, with 7-day AZA +/- 21-day VEN. In an expansion cohort of 26 patients (median age 71) with previously untreated European LeukemiaNet adverse-risk AML (50% TP53 mutated), triplet TAG-AZA-VEN induced response in 69% (n=18/26; 39% complete remission [CR], 19% complete remission with incomplete count recovery [CRi], 12% morphologic leukemia-free state [MLFS]). Among 13 patients with TP53 mutations, 7/13 (54%) achieved CR/CRi/MLFS (CR = 4, CRi = 2, MLFS = 1). Twelve of 17 (71%) tested responders had no flow measurable residual disease. Median overall survival and progression-free survival were 14 months (95% CI, 9.5-NA) and 8.5 months (95% CI, 5.1-NA), respectively. In summary, TAG-AZA-VEN shows encouraging safety and activity in high-risk AML, including TP53-mutated disease, supporting further clinical development of TAG combinations. The study was registered on ClinicalTrials.gov as #NCT03113643.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.A.L. received research support from AbbVie and Stemline Therapeutics. A.A.L. received consulting fees from Cimeio Therapeutics, IDRx, Jnana Therapeutics, ProteinQure, and Qiagen, and has equity as an adviser for Medzown. J.S.G. served in advisory role for AbbVie, Astellas, Bristol Myers Squibb, Genentech, Gilead and Servier and had trial institutional funding from AbbVie, Genentech, Pfizer, Prelude and AstraZeneca. M.S. served on an advisory board for Novartis, Kymera, Sierra Oncology, GlaxoSmithKline, and Rigel; consulted for Boston Consulting and Dedham group, and participated in medical education activities for Novartis, Curis Oncology, Haymarket Media, and Clinical Care Options. T.M. is a senior consultant for Stemline Therapeutics. C.B. and I.V.G. are employees of Stemline Therapeutics. N.P. received honoraria from Incyte, Novartis, LFB Biotechnologies, Stemline Therapeutics, Celgene, AbbVie, MustangBio, Roche Molecular Diagnostics, Blueprint Medicines, DAVA Pharmaceuticals, Springer, Aptitude Health, NeoPharm, and CareDX; has a consulting or advisory role in Blueprint Medicines, Pacylex, Immunogen, Bristol Myers Squibb, ClearView Healthcare Partners, Astellas Pharma, Protagonist Therapeutics, Triptych Health Partners, and CTI BioPharma Corp; received research funding from Novartis, Stemline Therapeutics, Samus Therapeutics, AbbVie, Cellectis, Affymetrix/Thermo Fisher Scientific, Daiichi Sankyo, Plexxikon, and MustangBio; received travel, accommodation, and other expenses from Stemline Therapeutics, Celgene, AbbVie, DAVA Oncology, and MustangBio. The remaining authors declare no competing financial interests.
Figures

References
-
- Muñoz L, Nomdedéu JF, López O, et al. Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica. 2001;86(12):1261–1269. - PubMed
-
- Testa U, Riccioni R, Militi S, et al. Elevated expression of IL-3Ralpha in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood. 2002;100(8):2980–2988. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

