Teclistamab impairs humoral immunity in patients with heavily pretreated myeloma: importance of immunoglobulin supplementation
- PMID: 38052042
- PMCID: PMC10787247
- DOI: 10.1182/bloodadvances.2023011658
Teclistamab impairs humoral immunity in patients with heavily pretreated myeloma: importance of immunoglobulin supplementation
Abstract
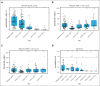
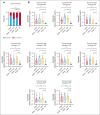
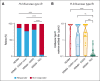
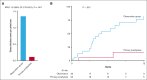
Teclistamab and other B-cell maturation antigen (BCMA)-targeting bispecific antibodies (BsAbs) have substantial activity in patients with heavily pretreated multiple myeloma (MM) but are associated with a high rate of infections. BCMA is also expressed on normal plasma cells and mature B cells, which are essential for the generation of a humoral immune response. The aim of this study was to improve the understanding of the impact of BCMA-targeting BsAbs on humoral immunity. The impact of teclistamab on polyclonal immunoglobulins and B cell counts was evaluated in patients with MM who received once-weekly teclistamab 1.5 mg/kg subcutaneously. Vaccination responses were assessed in a subset of patients. Teclistamabinduced rapid depletion of peripheral blood B cells in patients with MM and eliminated normal plasma cells in ex vivo assays. In addition, teclistamab reduced the levels of polyclonal immunoglobulins (immunoglobulin G [IgG], IgA, IgE, and IgM), without recovery over time while receiving teclistamab therapy. Furthermore, response to vaccines against Streptococcus pneumoniae, Haemophilus influenzae type B, and severe acute respiratory syndrome coronavirus 2 was severely impaired in patients treated with teclistamab compared with vaccination responses observed in patients with newly diagnosed MM or relapsed/refractory MM. Intravenous immunoglobulin (IVIG) use was associated with a significantly lower risk of serious infections among patients treated with teclistamab (cumulative incidence of infections at 6 months: 5.3% with IVIG vs 54.8% with observation only [P < .001]). In conclusion, our data show severe defects in humoral immunity induced by teclistamab, the impact of which can be mitigated by the use of immunoglobulin supplementation. This trial was registered at www.ClinicalTrials.gov as #NCT04557098.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.V.M. reports honoraria from, and membership on a board of directors/advisory committee for, Amgen, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Janssen Cilag, Pfizer, Sanofi, and Takeda; is a speaker bureau member for Janssen Cilag; and is a board of directors/advisory committee member for Oncopeptides. T.G.M. reports research funding (institutional) from Amgen, Janssen, Sanofi, and Seattle Genetics; and consultancy for GlaxoSmithKline and Roche. C.R. reports personal fees from Amgen, Bristol Myers Squibb, and Takeda. A.N. has served on advisory boards of, and received honorarium from, Adaptive Biotechnologies, Amgen, BeyondSpring, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Karyopharm, Oncopeptides, Pfizer, Sanofi, Secura Bio, and Takeda; has received grant/research support (institutional) from Aduro Biotech, Amgen, Arch Oncology, Bristol Myers Squibb, Genentech, GlaxoSmithKline, Janssen, Karyopharm, Kite Pharma, Pfizer, and Takeda; and received grant/research support for investigator-initiated studies from Amgen, GlaxoSmithKline, Janssen, Merck, and Takeda. A.B. is an employee of Janssen and is a stockholder in Janssen. K.C. is an employee of Janssen and is a stockholder in Janssen. A.P.-P. is an employee of Janssen and is a stockholder in Janssen. T.S. is an employee of Janssen and is a stockholder in Janssen. C.U. is an employee of Janssen and is a stockholder in Janssen. R.K. is an employee of Janssen and is a stockholder in Janssen. B.v.d.H. reports honoraria for data safety monitoring board membership from the Intergroupe Francophone du Myélome. S.K. serves on advisory boards for Janssen Pharmaceuticals. D.V. is an employee of Janssen and is a stockholder in Janssen. S.S. is an employee of Janssen and is a stockholder in Janssen. D.C.-S. is an employee of Janssen and is a stockholder in Janssen. M.D. is an employee of Janssen and is a stockholder in Janssen. S.Z. has received research funding from Celgene, Takeda, and Janssen; and serves on advisory boards for Janssen, Takeda, Bristol Myers Squibb, Oncopeptides, and Sanofi, all paid to institution. R.I.V. is an employee of Janssen and is a stockholder in Janssen. N.W.C.J.v.d.D. has received research support from Janssen Pharmaceuticals, Amgen, Celgene, Novartis, Cellectis, and Bristol Myers Squibb; and serves on advisory boards for Janssen Pharmaceuticals, AMGEN, Celgene, Bristol Myers Squibb, Takeda, Roche, Novartis, Bayer, Adaptive, Pfizer, AbbVie, and Servier, all paid to institution. The remaining authors declare no competing financial interest.
Figures

References
-
- van de Donk N, Zweegman S. T cell-engaging bispecific antibodies in cancer. Lancet. 2023;402(10396):142–158. - PubMed
-
- van de Donk NW, Moreau P, Garfall AL, et al. Long-term follow-up from MajesTEC-1 of teclistamab, a B cell maturation antigen (BCMA) x CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma (RRMM) J Clin Oncol. 2023;41(16 suppl) 8011-8011.
-
- Wong SW, Bar N, Paris L, et al. Alnuctamab (ALNUC; BMS-986349; CC-93269), a B cell maturation antigen (BCMA) x CD3 T cell engager (TCE), in patients (pts) with relapsed/refractory multiple myeloma (RRMM): results from a Phase 1 First-in-Human Clinical Study. Blood. 2022;140(suppl 1):400–402.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

