Mouse model of radiation retinopathy reveals vascular and neuronal injury
- PMID: 38052338
- PMCID: PMC11218432
- DOI: 10.1016/j.exer.2023.109729
Mouse model of radiation retinopathy reveals vascular and neuronal injury
Abstract
Purpose: To characterize the neuronal and vascular pathology in vivo and in vitro in a mouse model of radiation retinopathy.
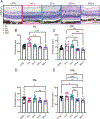
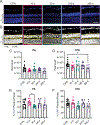
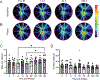
Methods: C57Bl/6J mice underwent cranial irradiation with 12 Gy and in vivo imaging by optical coherence tomography and of relative blood flow velocity by laser speckle flowgraphy for up to 3-6 months after irradiation. Retinal architecture, vascular density and leakage and apoptosis were analyzed by histology and immunohistochemistry before irradiation or at 10, 30, 240, and 365 days after treatment.
Results: The vascular density decreased in the plexiform layers starting at 30 days after irradiation. No impairment in retinal flow velocity was seen. Subtle perivascular leakage was present at 10 days, in particular in the outer plexiform layer. This corresponded to increased width of this layer. However, no significant change in the retinal thickness was detected by OCT-B scans. At 365 days after irradiation, the nuclear density was significantly reduced compared to baseline. Apoptosis was detected at 30 days and less prominent at 365 days.
Conclusions: By histology, vascular leakage at 10 days was followed by increased neuronal apoptosis and loss of neuronal and vascular density. However, in vivo imaging approaches that are commonly used in human patients did not detect pathology in mice.
Keywords: Apoptosis; Capillaries; Mouse; Radiation retinopathy.
Copyright © 2023. Published by Elsevier Ltd.
Figures



References
-
- ALT C, RUNNELS JM, MORTENSEN LJ, ZAHER W & LIN CP 2014. In vivo imaging of microglia turnover in the mouse retina after ionizing radiation and dexamethasone treatment. Invest Ophthalmol Vis Sci, 55, 5314–9. - PubMed
-
- AMOAKU WM, FREW L, MAHON GJ, GARDINER TA & ARCHER DB 1989. Early ultrastructural changes after low-dose X-irradiation in the retina of the rat. Eye (Lond), 3 (Pt 5), 638–46. - PubMed
-
- ARCHER DB, AMOAKU WM & GARDINER TA 1991. Radiation retinopathy--clinical, histopathological, ultrastructural and experimental correlations. Eye (Lond), 5 (Pt 2), 239–51. - PubMed
-
- AZIZ HA, SINGH N, BENA J, WILKINSON A & SINGH AD 2016. Vision Loss Following Episcleral Brachytherapy for Uveal Melanoma: Development of a Vision Prognostication Tool. JAMA Ophthalmol, 134, 615–20. - PubMed
-
- BARAKAT MR, SHUSTERMAN M, MOSHFEGHI D, DANIS R, GERTNER M & SINGH RP 2011. Pilot study of the delivery of microcollimated pars plana external beam radiation in porcine eyes. Arch Ophthalmol, 129, 628–32. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

