A Versatile Isocyanate-Mediated Strategy for Appending Chemical Tags onto Drug-Like Small Molecules
- PMID: 38052453
- PMCID: PMC10739574
- DOI: 10.1021/acs.bioconjchem.3c00352
A Versatile Isocyanate-Mediated Strategy for Appending Chemical Tags onto Drug-Like Small Molecules
Abstract
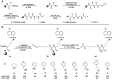
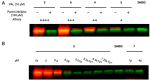
Target identification studies are a major hurdle in probe and drug discovery pipelines due to the need to chemically modify small molecules of interest, which can be time intensive and have low throughput. Here, we describe a versatile and scalable method for attaching chemical moieties to a small molecule, isocyanate-mediated chemical tagging (IMCT). By preparation of a template resin with an isocyanate capture group and a cleavable linker, nucleophilic groups on small molecules can be modified with an enforced one-to-one stoichiometry. We demonstrate a small molecule substrate scope that includes primary and secondary amines, thiols, phenols, benzyl alcohols, and primary alcohols. Cheminformatic analyses predict that IMCT is reactive with more than 25% of lead-like compounds in publicly available databases. To demonstrate that the method can produce biologically active molecules, we generated FKBP12 photoaffinity labeling (PAL) compounds with a wide range of affinities and showed that purified and crude cleavage products can bind to and label FKBP12. This method could be used to rapidly modify small molecules for many applications, including the synthesis of PAL probes, fluorescence polarization probes, pull-down probes, and degraders.
Conflict of interest statement
The authors declare the following competing financial interest(s): C.C.H., J.A.K., C.C., C.M.W., and A.N.K. have filed a patent (US 20220089537) related to this project.
Figures

References
-
- Sannino A.; Gironda-Martínez A.; Gorre E. M. D.; Prati L.; Piazzi J.; Scheuermann J.; Neri D.; Donckele E. J.; Samain F. Critical Evaluation of Photo-Cross-Linking Parameters for the Implementation of Efficient DNA-Encoded Chemical Library Selections. ACS Comb. Sci. 2020, 22 (4), 204–212. 10.1021/acscombsci.0c00023. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

